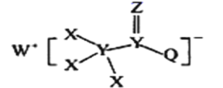
ЬтФПФкШн
ЁОЬтФПЁПЃЈ1ЃЉЛЏбЇЗДгІ2A(g)+B(?)![]() 2C(g)ДяЕНЛЏбЇЦНКтЪБЃК
2C(g)ДяЕНЛЏбЇЦНКтЪБЃК
ЂйЩ§ИпЮТЖШЪБЃЌCЕФСПМѕЩйЃЌдђЗДгІЮяЕФФмСПзмКЭ____________ЩњГЩЮяЕФФмСПзмКЭЃЈЬюЁА>ЁБЁЂЁА<ЁБЛђЁА=ЁБЃЉЁЃ
ЂкШчЙћдіДѓбЙЧПЃЌЦНКтЯђе§ЗДгІЗНЯђвЦЖЏЃЌдђBЪЧ____________ЬЌЮяжЪЁЃ
ЂлШєдіМгBЕФЮяжЪЕФСПЃЌЦНКтВЛвЦЖЏЃЌЫЕУїBЪЧ____________ЬЌЮяжЪЁЃ
ЃЈ2ЃЉШчЭМЃЌдкКубЙУмБеШнЦїжаМгШы2 mol AКЭ2 mol BЃЌЦ№ЪМЪБШнЦїЬхЛ§ЮЊV LЃЌЗЂЩњШчЯТЗДгІВЂДяЕНЛЏбЇЦНКтзДЬЌЃК2 A(g)ЃЋB(g)![]() x C(g)ЁЃЦНКтЪБЃЌAЁЂBЁЂCЕФЮяжЪЕФСПжЎБШЮЊ1ЁУ3ЁУ4ЃЌCЕФЮяжЪЕФСПЮЊ y molЁЃ
x C(g)ЁЃЦНКтЪБЃЌAЁЂBЁЂCЕФЮяжЪЕФСПжЎБШЮЊ1ЁУ3ЁУ4ЃЌCЕФЮяжЪЕФСПЮЊ y molЁЃ
 ИљОнЬтжаЪ§ОнМЦЫуЃЌx ЃН_________ЁЂy ЃН_________ЃЛ
ИљОнЬтжаЪ§ОнМЦЫуЃЌx ЃН_________ЁЂy ЃН_________ЃЛ
ЁОД№АИЁПЃО Цј ЙЬЛђвК 2 1.6
ЁОНтЮіЁП
ЃЈ1ЃЉЂйЩ§ИпЮТЖШЪБЃЌCЕФСПМѕЩйЃЌЫЕУїЩ§ЮТЦНКтЯђФцЯђвЦЖЏЃЌЫЕУїИУЗДгІЕФе§ЗДгІЪЧЗХШШЗДгІЃЌЫљвдЗДгІЮяЕФФмСПзмКЭДѓгкЩњГЩЮяЕФФмСПзмКЭЃЌЙЪЬюЃОЃЛ
ЂкШчЙћдіДѓбЙЧПЃЌЦНКтЯђе§ЗДгІЗНЯђвЦЖЏЃЌЫЕУїИУЗДгІЪЧЦјЬхЮяжЪЕФСПжЎКЭМѕаЁЕФЗДгІЃЌЫљвдBЪЧЦјЬхЃЌЙЪД№АИЮЊЃКЦјЃЛ
ЂлШєдіМгBЕФЮяжЪЕФСПЃЌЦНКтВЛвЦЖЏЃЌдђЫЕУїBЪЧЙЬЛђвКЬЌЮяжЪЃЛ
ЃЈ2ЃЉЦ№ЪМЪБдкКубЙУмБеШнЦїжаМгШы2 mol AКЭ2 mol BЃЌЗЂЩњШчЯТЗДгІ2 A(g)ЃЋB(g)![]() x C(g)ЁЃЦНКтЪБЃЌAЁЂBЁЂCЕФЮяжЪЕФСПжЎБШЮЊ1ЁУ3ЁУ4ЃЌCЕФЮяжЪЕФСПЮЊ y molЁЃЩшБфЛЏЕФBЕФЮяжЪЕФСПЮЊbЃЌдђБфЛЏЕФAЕФЮяжЪЕФСПЮЊ2bЃЌБфЛЏЕФCЕФЮяжЪЕФСПЮЊxbЃЌПЩСаШ§ЖЮЪНЃК
x C(g)ЁЃЦНКтЪБЃЌAЁЂBЁЂCЕФЮяжЪЕФСПжЎБШЮЊ1ЁУ3ЁУ4ЃЌCЕФЮяжЪЕФСПЮЊ y molЁЃЩшБфЛЏЕФBЕФЮяжЪЕФСПЮЊbЃЌдђБфЛЏЕФAЕФЮяжЪЕФСПЮЊ2bЃЌБфЛЏЕФCЕФЮяжЪЕФСПЮЊxbЃЌПЩСаШ§ЖЮЪНЃК
2A(g)+B(g)![]() 2C(g)
2C(g)
Ц№ЪМЃЈmolЃЉ 2 2 0
БфЛЏЃЈmolЃЉ 2b b xb
ЦНКтЃЈmolЃЉ 2-2b 2-b xb
xb=yЃЌ(2-2b):(2-b):xb=1:3:4ЃЌЧѓЕУb=0.8ЃЌx=2ЃЌy=1.6ЁЃЙЪД№АИЮЊЃК2ЃЌ1.6ЁЃ

ЁОЬтФПЁПдк2LЕФУмБеШнЦїжаЃЌНјааШчЯТЛЏбЇЗДгІЃКCO2ЃЈgЃЉЃЋH2ЃЈgЃЉ![]() COЃЈgЃЉЃЋH2OЃЈgЃЉЃЌЦфЛЏбЇЦНКтГЃЪ§KКЭЮТЖШtЕФЙиЯЕШчЯТБэЃК
COЃЈgЃЉЃЋH2OЃЈgЃЉЃЌЦфЛЏбЇЦНКтГЃЪ§KКЭЮТЖШtЕФЙиЯЕШчЯТБэЃК
tЁц | 600 | 800 | 830 | 1000 | 1200 |
K | 0.25 | 0.9 | 1.0 | 1.7 | 2.6 |
ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉИУЗДгІЕФЛЏбЇЦНКтГЃЪ§БэДяЪНЮЊK ЃН ЁЃ
ЃЈ2ЃЉИУЗДгІЮЊ ЗДгІЃЈбЁЬюЁАЮќШШЁБЁЂЁАЗХШШЁБЃЉЁЃЗДгІДяЦНКтКѓЃЌШєдйЭЈШывЛЖЈСПCO2ЃЌдђЦНКтГЃЪ§KНЋ________ЃЌCO2ЕФзЊЛЏТЪ________ЁЃ(ЬюЁАдіДѓЁБЁЂЁАМѕаЁЁБЛђЁАВЛБфЁБ)
ЃЈ3ЃЉФмХаЖЯИУЗДгІЪЧЗёДяЕНЛЏбЇЦНКтзДЬЌЕФвРОнЪЧ ЃЈЖрбЁПлЗжЃЉЁЃ
aЃЎШнЦїжабЙЧПВЛБф bЃЎЛьКЯЦјЬхжаc(CO)ВЛБф cЃЎvе§(H2)ЃНvФц(H2O) dЃЎc(CO2)ЃНc(CO)
ЃЈ4ЃЉШє 600ЁцЪБЃЌЯђШнЦїжаГфШы1mol COЁЂ1mol H2OЃЌЗДгІДяЕНЦНКтКѓЃЌCOЕФзЊЛЏТЪЪЧ ЁЃ