ЬтФПФкШн
ЁОЬтФПЁПИпУЬЫсМиОпгаЧПбѕЛЏадЃЌЙуЗКгУгкЛЏЙЄЁЂвНвЉЁЂВЩПѓЁЂН№ЪєжЮСЖМАЛЗОГБЃЛЄСьгђЕШЁЃKMnO4ЕФжЦБИЪЧвдЖўбѕЛЏУЬ(MnO2)ЮЊдСЯЃЌдкЧПМюадНщжЪжаБЛбѕЛЏЩњГЩФЋТЬЩЋЕФУЬЫсМи(K2MnO4)ЃЛШЛКѓдквЛЖЈpHЯТK2MnO4ЦчЛЏЩњГЩзЯЩЋKMnO4ЁЃЛиД№ЯТСаЮЪЬт
(1)K2MnO4ЕФжЦБИ
ЪЕбщВНжш | ЯжЯѓЛђНтЪЭ |
ЂйГЦШЁ2.5 gKClO3ЙЬЬхКЭ5.2gKOHЙЬЬхжУгкЬњлсліжаЃЌМгШШШлШк | ВЛгУДЩлсліЕФдвђЪЧ____________ЁЃ |
ЂкЗжЖрДЮМгШы3gMnO2ЙЬЬх | ВЛвЛДЮМгШыЕФдвђЪЧ__________________ЁЃ |
(2)KMnO4ЕФжЦБИ
ГУШШЯђK2MnO4ШмвКжаМгШы1 mol/L H3PO4ШмвКЃЌжБжСK2MnO4ШЋВПЦчЛЏЃЌХаЖЯШЋВПЦчЛЏЕФЗНЗЈЪЧгУВЃСЇАєеКШЁШмвКгкТЫжНЩЯЃЌЯжЯѓЮЊ_________________________ЁЃШЛКѓГУШШЙ§ТЫЃЌНЋТЫвКЕЙШыеєЗЂУѓжаМгШШЕНвКУцГіЯжОЇФЄЃЌГфЗжРфШДКѓЙ§ТЫЃЌдк80ЁцКцЯфжаИЩдя3hЃЌВЛбЁгУИќИпЮТЖШЕФдвђЪЧ_____________________________ЁЃ
(3)ВњЦЗЗжЮі
i.ВЛЭЌpHЯТВњЦЗжаKMnO4КЌСП
МгШыH3PO4ЬхЛ§ЃЏmL | ШмвКЕФpH | ВњЦЗжЪСП | KMnO4жЪСП | KMnO4жЪСПЗжЪ§ |
10.50 | 12.48 | 2.35 | 2.05 | 87.23 |
12.50 | 11.45 | 2.45 | 2.18 | 88.98 |
14.50 | 10.89 | 2.18 | 1.87 | 85.78 |
16.50 | 10.32 | 2.28 | 1.75 | 76.75 |
18.50 | 9.44 | 2.09 | 1.48 | 70.81 |
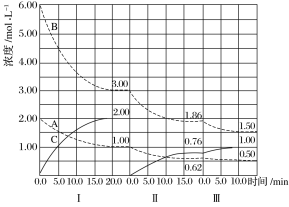
ДгБэИёжаЪ§ОнЗжЮіЃЌдкЦчЛЏЪБбЁдёШмвКЕФзюМбpHЪЧ________________ЁЃ
ii.РћгУH2C2O4БъзМШмвКВтЖЈKMnO4ЕФДПЖШЁЃВтЖЈВНжшШчЯТЃК
ЂйШмвКХфжЦЃКГЦШЁ1.000gЕФKMnO4ЙЬЬхбљЦЗЃЌЗХШы_____________жаШмНтЃЌШЛКѓРфжСЪвЮТКѓШЋВПзЊвЦЕН100mLШнСПЦПжаЃЌМгеєСѓЫЎжСПЬЖШЯпЁЃ
ЂкЕЮЖЈЃКвЦШЁ25 mLKMnO4ШмвКгкзЖаЮЦПжаЃЌМгЩйСПСђЫсЫсЛЏЃЌгУ0.1400mol/LЕФH2C2O4БъзМШмвКЕЮЖЈЃЌЗЂЩњЗДгІЃК2MnO4-ЃЋ5H2C2O4ЃЋ6H+ЃН2Mn2ЃЋЃЋ10C02ЃЋ8H2OЃЌЕБШмвКзЯЩЋЭЪЩЋЧвАыЗжжгФкВЛБфЩЋМДЮЊжеЕуЃЌЦНааЕЮЖЈ3ДЮЃЌH2C2O4ШмвКЕФЦНОљгУСПЮЊ23.90mLЃЌдђбљЦЗДПЖШЮЊ_______________ЃЅ(БЃСє1ЮЛаЁЪ§)ЁЃ
ЁОД№АИЁПДЩлсліжаЕФSiO2гыKOHЗДгІ ЪЙMnO2ГфЗжЗДгІЃЌЬсИпЦфзЊЛЏТЪЛђепРћгУТЪ ГЪЯжзЯЩЋЃЌЖјВЛЪЧФЋТЬЩЋ ЗРжЙKMnO4ЪмШШЗжНт 11.45 ЩеБ 84.6%
ЁОНтЮіЁП
(1) ЂйДЩлслідСЯКЌгаSiO2ЃЌЪЧЫсадбѕЛЏЮяЃЌФмКЭМюЗДгІЃЛ
ЂкЗжЖрДЮМгШы3gMnO2ЙЬЬхЃЌПЩЬсИпдСЯРћгУТЪЃЛ
(2) ИљОнЁАK2MnO4ШмвКЯдТЬЩЋЁБПЩжЊЃЌШчЙћИУЦчЛЏЗДгІНсЪјЃЌдђЗДгІКѓЕФШмвКВЛЛсЯдЪОТЬЩЋЃЛKMnO4дкМгШШЬѕМўЯТЗжНтЩњГЩK2MnO4ЃЛ
(3) i.ЗжЮіБэжаЪ§ОнбЁдёВњЦЗжаKMnO4жЪСПЗжЪ§зюИпЪБЖдгІШмвКЕФpHЃЛ
ii.ЂйШмвКХфжЦЪБЙЬЬхШмНтдкЩеБжаНјааЃЛ
ЂкЕЮЖЈЪБЯћКФ23.90mL0.1400mol/LЕФH2C2O4БъзМШмвКЃЌдђВЮМгЗДгІЕФH2C2O4ЕФЮяжЪЕФСПЮЊ0.0239LЁС0.1400mol/L=3.346ЁС10-3molЃЌИљОнЗДгІЃК2MnO4-ЃЋ5H2C2O4ЃЋ6H+ЃН2Mn2ЃЋЃЋ10CO2ЃЋ8H2OПЩжЊВЮМгЗДгІЕФKMnO4ЕФЮяжЪЕФСПЮЊ3.346ЁС10-3molЁС![]() =1.3348ЁС10-3molЃЌОнДЫМЦЫубљЦЗДПЖШЁЃ
=1.3348ЁС10-3molЃЌОнДЫМЦЫубљЦЗДПЖШЁЃ
(1) ЂйДЩлслідСЯКЌгаSiO2ЃЌдкИпЮТЯТЃЌДЩлсліПЩвдКЭKOHЗЂЩњЗДгІSiO2+2KOH![]() K2SiO3+H2OЃЌИЏЪДДЩлсліЃЌЙЪВЛФмЪЙгУДЩлсліЃЌЖјЪЙгУЬњлсліЃЛ
K2SiO3+H2OЃЌИЏЪДДЩлсліЃЌЙЪВЛФмЪЙгУДЩлсліЃЌЖјЪЙгУЬњлсліЃЛ
ЂкЮЊЪЙMnO2ГфЗжЗДгІЃЌЬсИпЦфзЊЛЏТЪЃЌМгШыMnO2ЪБПЩЗжХњМгШыЃЌЖјВЛвЛДЮадМгШыЃЛ
(2) гЩгкK2MnO4ШмвКЯдТЬЩЋЃЌЫљвдгУВЃСЇАєеКШЁШ§ОБЩеЦПФкЕФШмвКЕудкТЫжНЩЯЃЌШєТЫжНЩЯжЛгазЯКьЩЋКлМЃЃЌЮоТЬЩЋКлМЃЃЌБэУїЗДгІвбЦчЛЏЭъШЋЃЛОЇЬхКцИЩЪБгІПижЦЮТЖШВЛГЌЙ§80ЁцЃЌвдЗРжЙKMnO4ЪмШШЗжНтЃЛ
(3) i.гЩБэжаЪ§ОнПЩжЊЃЌЦчЛЏЪБбЁдёШмвКЕФpHЮЊ11.45ЪБЫљЕУВњЦЗЕФжЪСПзюИпЃЌВњЦЗжаKMnO4жЪСПЗжЪ§ЕФзюИпЃЌЙЪПижЦШмвКЕФзюМбpHЪЧ11.45ЃЛ
ii.ЂйХфжЦKMnO4ШмвКЪБЃЌГЦШЁЕФKMnO4ЙЬЬхбљЦЗЃЌгІЗХШыЩеБжаШмНтЃЌШЛКѓРфжСЪвЮТКѓШЋВПзЊвЦЕН100mLШнСПЦПжаЃЌМгеєСѓЫЎжСПЬЖШЯпЃЛ
ЂкЕЮЖЈЪБЯћКФ23.90mL0.1400mol/LЕФH2C2O4БъзМШмвКЃЌдђВЮМгЗДгІЕФH2C2O4ЕФЮяжЪЕФСПЮЊ0.0239LЁС0.1400mol/L=3.346ЁС10-3molЃЌИљОнЗДгІЃК2MnO4-ЃЋ5H2C2O4ЃЋ6H+ЃН2Mn2ЃЋЃЋ10CO2ЃЋ8H2OПЩжЊВЮМгЗДгІЕФKMnO4ЕФЮяжЪЕФСПЮЊ3.346ЁС10-3molЁС![]() =1.3348ЁС10-3molЃЌдђбљЦЗДПЖШ=
=1.3348ЁС10-3molЃЌдђбљЦЗДПЖШ= Ёж84.6%ЁЃ
Ёж84.6%ЁЃ

 ЧЇРяТэзпЯђМйЦкЦкФЉЗТецЪдОэКЎМйЯЕСаД№АИ
ЧЇРяТэзпЯђМйЦкЦкФЉЗТецЪдОэКЎМйЯЕСаД№АИЁОЬтФПЁПбЁдёзАжУЃЌЭъГЩЪЕбщЁЃ
|
|
|
|
Ђй | Ђк | Ђл | Ђм |
(1)ХчШЊЪЕбщЃЌжЄУїАБЦјМЋвзШмгкЫЎЃЌбЁгУ__(ЬюађКХЃЌЯТЭЌ)ЁЃ
(2)ХфжЦ100mL0.1molЁЄL-1NaOHШмвКЃЌбЁгУ__ЁЃ
(3)МјБ№Na2CO3КЭNaHCO3ЙЬЬхЃЌбЁгУ__ЁЃ
(4)гУгкЗжРыФрЩГгыЪГбЮШмвКЃЌбЁгУ__ЁЃ