��Ŀ����
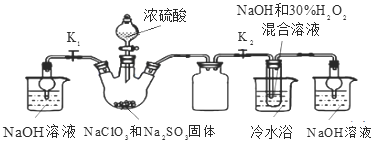
����Ŀ������������(NOSO4H)��Ҫ����Ⱦ�ϡ�ҽҩ�ȹ�ҵ��ʵ����������ͼװ��(�г�װ����)�Ʊ�����NOSO4H�����ⶨ��Ʒ�Ĵ��ȡ�
��֪��NOSO4H ��ˮ�ֽ⣬������Ũ��������ֽ⡣
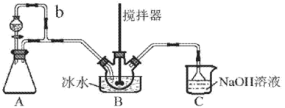
(1)װ��A ���� Na2SO3 ��������ȡ SO2
��A �з�Ӧ�Ļ�ѧ����ʽΪ___________________________��
�ڵ��� b ��������_____________________��
(2)װ��C ����Ҫ������ (�����ӷ���ʽ��ʾ)_____________________��
(3)��ʵ��װ�ô��ڿ��ܵ��� NOSO4H �������͵�ȱ����___________________________��
(4)�ⶨ����������NOSO4H �Ĵ��ȣ�ȷ��ȡ 1.380g ��Ʒ���� 250mL �ĵ���ƿ�У����� 0.1000mol��L-1��60.00mL �� KMnO4 ��Һ��10mL25%H2SO4 ��Һ��ҡ�ȡ�Ȼ�� 0.2500mol��L-1 ������(Na2C2O4)��Һ�������ƿ�У����IJ�������Һ�����Ϊ 20.00mL��
��֪��2KMnO4+5NOSO4H+2H2O=K2SO4+2MnSO4+5HNO3+2H2SO4
����ƽ��_______![]() + ______
+ ______![]() +_______=_____________Mn2++____CO2��+ _______H2O
+_______=_____________Mn2++____CO2��+ _______H2O
������������Ĵ���=__________(��ȷ�� 0.1%)(д���������)
���𰸡�Na2SO3+H2SO4=Na2SO4+H2O+SO2�� ƽ��ѹǿʹ��Һ©����Һ��˳������ SO2+2OH-=![]() +H2O C ��ˮ�����B �е���NOSO4H �ֽ� 2 5 16H+ 2 10 8 92.0%
+H2O C ��ˮ�����B �е���NOSO4H �ֽ� 2 5 16H+ 2 10 8 92.0%
��������
����װ��A����ȡSO2�ģ�д����ѧ����ʽ�����ݵ���b�����ӷ�ʽ֪����ƿ�ͷ�Һ©����ѹǿ��ȣ�����SO2���ж������壬β��Ҫ������������Ŀ��NOSO4H ��ˮ�ֽ⣬�������Ϣ���ж�ȱ����ˮװ�ã�����C2O42-���������֮��Ӧ��KMnO4��������KMnO4�����NOSO4H�������������Ʒ�Ĵ��ȡ�
(1)��װ��A�������������ƺ�Ũ���ᷴӦ��ȡSO2����Ӧ�Ļ�ѧ����ʽ��Na2SO3+H2SO4=Na2SO4+SO2��+H2O����ΪNa2SO3+H2SO4=Na2SO4+SO2��+H2O��
�ڵ���b�������ǣ�ƽ��ѹǿʹ��Һ©���е�Һ��˳�����£���Ϊƽ��ѹǿʹ��Һ©���е�Һ��˳�����¡�
(2)װ��C����Ҫ��������������������Һ���ն��������ֹ��Ⱦ��������Ӧ�����ӷ���ʽ��SO2+2OH-=SO32-+H2O����Ϊ��SO2+2OH-=SO32-+H2O��
(3)��ʵ��װ�ô��ڿ��ܵ���NOSO4H�������͵�ȱ���ǣ�Cװ���е�ˮ���������װ��B��ʹNOSO4Hˮ�⣬Ӧ��BC������һ��װ��Ũ�����ϴ��ƿ����ΪCװ���е�ˮ���������װ��B��ʹNOSO4Hˮ�⡣
(4)������Һ�и�����غͲ����Ʒ���������ԭ��Ӧ�����ɶ�����̼����Ԫ�ػ��ϼ�+7�۽��͵�+2�ۣ�����ת��5e-��̼Ԫ�ػ��ϼ�+3�۱仯Ϊ+4�ۣ�����ת��e-�������غ㡢ԭ���غ㡢����غ���ƽ�õ����ӷ���ʽ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O����Ϊ2��5��16H+��2��10��8��
����MnO4-��C2O42-��Ӧ�����ʵ���Ϊn1mol��
2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O
![]() n1=0.002mol��
n1=0.002mol��
����NOSO4H��Ӧ�ĸ���������ʵ���=0.1000molL-1��0.0600L-0.002mol=0.004mol��
���������������ʵ���Ϊn2mol��
2KMnO4+5NOSO4H+2H2O=K2SO4+2MnSO4+5HNO3+2H2SO4��
![]() n2=0.01mol��
n2=0.01mol��
����������Ĵ���= ![]() ��100%=92.0%��
��100%=92.0%��
�ʴ�Ϊ��92.0%��

 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�