��Ŀ����
þ����Ͻ�����;�㷺�Ľ������ϣ��Ӻ�ˮ����ȡþ����Ҫ�������£�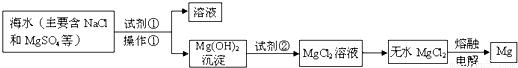
��ش�
��1�����н����У���ұ��������þ��ͬ����______������ţ���
a��Na b��Fe c��Al d��Hg
��2������ʵ������ɲ����٣������õ��IJ���������______��
��3���Լ�����Mg��OH��2��Ӧ�����ӷ���ʽ��______��
��4����ʵ�����У������Լ����������ӵIJ���������ʵ������Ϊ��ȡ�����Լ������Թ��У�______����֤���Լ����к��и������ӣ�
���𰸡���������1�����ݽ���ұ����ԭ���ͷ��������жϣ��ȷֽⷨ�������ڲ����õĽ������繯���������������Ƶã����Ȼ�ԭ�����û�ԭ������������̿��һ����̼�����ý����ȣ���ԭ�����������������������ȷ�Ӧ������ȡһЩ�۵�ߵĽ����ǿ�������������������ý�̿����ⷨ����ⷨұ������Ĵ����ĵ��ܳɱ��ϸߣ���ұ���Ľ������ȸ�һ�����ڽ����ľ�����������K��Ca��Na��Mg��Al�Ȼ��ý�����������������CuSO4+Fe�TCu+FeSO4Na+KCl�TK+NaCl����Ӧ�����Ǹ��£���գ���ԭ���Ǹ߷е�����Ƶͷе������
��2���ٲ����Ƿ����������þ���������ù��˲������õ��IJ�������Ϊ��©�����ձ�����������
��3���Լ���Ϊ������Mg��OH��2��Ӧ�����Ȼ�þ��ˮ��
��4�������Լ������������Ǽ��������ӣ��Լ��������ữ����������������ɫ����֤����
����⣺��1�����ݽ���ұ����ԭ���ͷ�����a�������õ�������Ȼ����Ʊ���b��������������������ͻ�ԭ����Ӧ���ɣ�c�����ǵ�������������Ʊ���d�����������ȷֽⷨ�Ʊ���
�ʴ�Ϊ��ac��
��2���ٲ����Ƿ����������þ���������ù��˲������õ��IJ�������Ϊ��©�����ձ������������ʴ�Ϊ��©�����ձ�����������
��3���Լ���Ϊ������Mg��OH��2��Ӧ�����Ȼ�þ��ˮ�����ӷ���ʽΪ��Mg��OH��2+2H+=Mg2++2H2O���ʴ�Ϊ��Mg��OH��2+2H+=Mg2++2H2O��
��4�������Լ������������Ǽ��������ӣ��Լ��������ữ����������������ɫ����֤�����ʴ�Ϊ�����������ữ�����������ɰ�ɫ������
���������⿼���˽���ұ����ԭ��Ӧ�ã����˲��������գ����ʵ��ᴿ�����Ӽ��鷽�����жϣ���Ŀ�Ѷ��еȣ�
��2���ٲ����Ƿ����������þ���������ù��˲������õ��IJ�������Ϊ��©�����ձ�����������
��3���Լ���Ϊ������Mg��OH��2��Ӧ�����Ȼ�þ��ˮ��
��4�������Լ������������Ǽ��������ӣ��Լ��������ữ����������������ɫ����֤����
����⣺��1�����ݽ���ұ����ԭ���ͷ�����a�������õ�������Ȼ����Ʊ���b��������������������ͻ�ԭ����Ӧ���ɣ�c�����ǵ�������������Ʊ���d�����������ȷֽⷨ�Ʊ���
�ʴ�Ϊ��ac��
��2���ٲ����Ƿ����������þ���������ù��˲������õ��IJ�������Ϊ��©�����ձ������������ʴ�Ϊ��©�����ձ�����������
��3���Լ���Ϊ������Mg��OH��2��Ӧ�����Ȼ�þ��ˮ�����ӷ���ʽΪ��Mg��OH��2+2H+=Mg2++2H2O���ʴ�Ϊ��Mg��OH��2+2H+=Mg2++2H2O��
��4�������Լ������������Ǽ��������ӣ��Լ��������ữ����������������ɫ����֤�����ʴ�Ϊ�����������ữ�����������ɰ�ɫ������
���������⿼���˽���ұ����ԭ��Ӧ�ã����˲��������գ����ʵ��ᴿ�����Ӽ��鷽�����жϣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
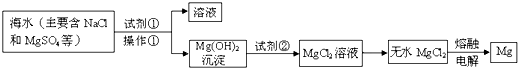
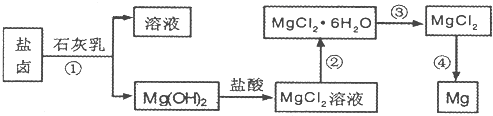
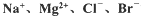 �ȣ���ģ�ҵ��������ȡþ����Ҫ�������£��ش��������⣺
�ȣ���ģ�ҵ��������ȡþ����Ҫ�������£��ش��������⣺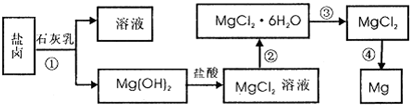
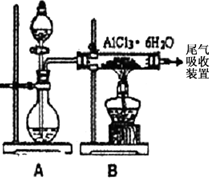

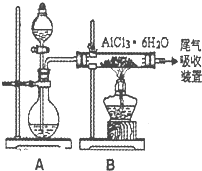
 ��ˮAlCl3ʵ��װ��ͼ��װ��A�е���Һ��ֱ���
��ˮAlCl3ʵ��װ��ͼ��װ��A�е���Һ��ֱ���