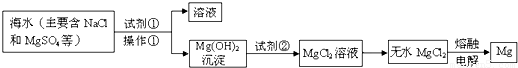
题目内容
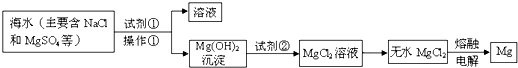
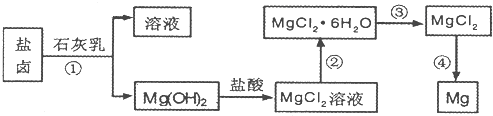
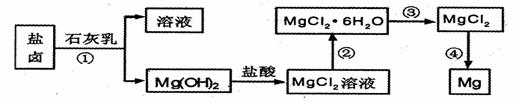
镁及其合金是用途广泛的金属材料,目前世界上60%的镁是从海水中提取的。.某学校课外兴趣小组从海水晒盐后的盐卤(主要含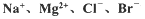 等)中模拟工业生产来提取镁,主要过程如下,回答下列问题:
等)中模拟工业生产来提取镁,主要过程如下,回答下列问题:
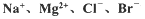 等)中模拟工业生产来提取镁,主要过程如下,回答下列问题:
等)中模拟工业生产来提取镁,主要过程如下,回答下列问题: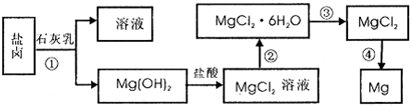
(1)从转化①得到的Mg(OH)2沉淀中混有少量的Ca(OH)2,除去少量Ca(OH)2的方法是先将沉淀加入到盛有_________的烧杯中,充分搅拌后经_________、_________(填操作方法)干燥可得纯净的Mg(OH)2,在此操作过程中,玻璃棒的作用是搅拌和__________
(2)写出转化④中发生反应的化学方程式__________________
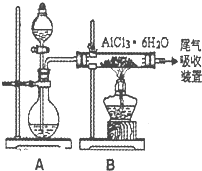
(3)已知转化③的反应原理与制取无水AlCl3相同,下图是制取无水AlCl3实验装置图。装置A中的两液体分别是浓硫酸和浓盐酸。请回答:
(2)写出转化④中发生反应的化学方程式__________________
(3)已知转化③的反应原理与制取无水AlCl3相同,下图是制取无水AlCl3实验装置图。装置A中的两液体分别是浓硫酸和浓盐酸。请回答:

①为什么不直接用加热方法来制取无水AlCl3,请用化学方式表示:_________;
②分液漏斗中应盛装的试剂是_________;
③由分液漏斗向烧瓶中加试剂时应注意的事项是_________
(4)含有铝元素的另一物质明矾作为膨化剂炸油条(饼)或膨化食品时,若在面粉里加入小苏打后,再加入明矾,则会使等量的小苏打释放出比单放小苏打多一倍的二氧化碳,这样就可以使油条(饼)在热油锅中一下子就鼓起来,得到香脆可口的油条(饼)了。请用化学反应方程式解释得到香脆可口的油条(饼)的原因:_____________________。
②分液漏斗中应盛装的试剂是_________;
③由分液漏斗向烧瓶中加试剂时应注意的事项是_________
(4)含有铝元素的另一物质明矾作为膨化剂炸油条(饼)或膨化食品时,若在面粉里加入小苏打后,再加入明矾,则会使等量的小苏打释放出比单放小苏打多一倍的二氧化碳,这样就可以使油条(饼)在热油锅中一下子就鼓起来,得到香脆可口的油条(饼)了。请用化学反应方程式解释得到香脆可口的油条(饼)的原因:_____________________。
(1)饱和MgCl2(或氯化镁)溶液;过滤;洗涤;引流
(2)MgCl2 Mg+Cl2↑
Mg+Cl2↑
(3)① AlCl3+3H2O=Al(OH)3+3HCl↑(写成AlCl3+3H2O==Al(OH)3+3HCl↑、2 Al(OH)3==Al2O3+3H2O或写成2AlCl3+3H2O==Al2O3+6HCl↑也可以);② 浓盐酸;③控制分液漏斗活塞,使浓盐酸缓缓加入到浓硫酸中
(4)Al3++3HCO3-= Al(OH)3↓+3CO2↑
(2)MgCl2
 Mg+Cl2↑
Mg+Cl2↑(3)① AlCl3+3H2O=Al(OH)3+3HCl↑(写成AlCl3+3H2O==Al(OH)3+3HCl↑、2 Al(OH)3==Al2O3+3H2O或写成2AlCl3+3H2O==Al2O3+6HCl↑也可以);② 浓盐酸;③控制分液漏斗活塞,使浓盐酸缓缓加入到浓硫酸中
(4)Al3++3HCO3-= Al(OH)3↓+3CO2↑

练习册系列答案
 同步轻松练习系列答案
同步轻松练习系列答案 课课通课程标准思维方法与能力训练系列答案
课课通课程标准思维方法与能力训练系列答案
相关题目
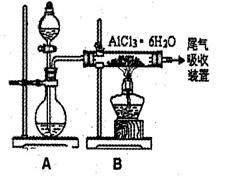 无水AlCl3实验装置图。装置A中的两液体分别是
无水AlCl3实验装置图。装置A中的两液体分别是