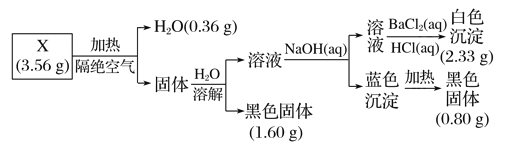
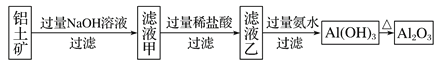
��Ŀ����
����Ŀ���������������ʵ���Һ����NaCl ��NH4Cl ��Na2CO3 ��Al2(SO4)3 ��CH3COOH ��NaHCO3
��1��25��ʱ��0.1mol��L-1����Һ��______�ԣ�0.1mol��L-1����Һ��pH________7���>������������<��������ԭ����______________________________________�������ӷ���ʽ��ʾ����
��2�����£��>������������<�����£�Ũ�Ⱦ�Ϊ0.1mol/L�Ģۺ͢���Һ����������________�����ͬ�����ǡ�����ͬ��������Һ��pH����_________�ޣ��>������������<������
��3��������Һ�������ɲ��������յõ���������____________���ѧʽ����
��4��������0.1 mol/L�Ģ���Һ��ˮϡ�����У����б���ʽ������һ��������_________��
A��c(H��) B�� 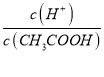 C��c(H��)��c(OH��)
C��c(H��)��c(OH��)
���𰸡� �� < NH4++H2O![]() NH3��H2O+H+ ��ͬ > Al2(SO4)3 B
NH3��H2O+H+ ��ͬ > Al2(SO4)3 B
����������1���Ȼ��Ʋ�ˮ�⣬25��ʱ��0.1mol��L-1�Ȼ�����Һ�����ԣ�笠�ˮ�⣬��0.1mol��L-1�Ȼ����Һ��pH��7��笠�ˮ������ӷ���ʽΪNH4++H2O![]() NH3��H2O+H+����2��̼������̼���ˮ�⣬��ˮ��ֲ����У�̼�������е�̼��������Ӵ���ˮ��ƽ��͵���ƽ�⣬��Ũ�Ⱦ�Ϊ0.1mol/L������Һ������������ͬ������̼�����ˮ��̶ȴ���̼��������ӣ�����ҺŨ�����ʱ̼������Һ�ļ���ǿ��̼�����ƣ�pH���ۣ��ޣ���3��������ˮ�⣬ˮ�����ȣ����������ɵ��������ѻӷ����ᣬ����������Һ�������ɲ��������յõ���������Ȼ��Al2(SO4)3����4�����������ᣬ���ڵ���ƽ�⣺CH3COOH
NH3��H2O+H+����2��̼������̼���ˮ�⣬��ˮ��ֲ����У�̼�������е�̼��������Ӵ���ˮ��ƽ��͵���ƽ�⣬��Ũ�Ⱦ�Ϊ0.1mol/L������Һ������������ͬ������̼�����ˮ��̶ȴ���̼��������ӣ�����ҺŨ�����ʱ̼������Һ�ļ���ǿ��̼�����ƣ�pH���ۣ��ޣ���3��������ˮ�⣬ˮ�����ȣ����������ɵ��������ѻӷ����ᣬ����������Һ�������ɲ��������յõ���������Ȼ��Al2(SO4)3����4�����������ᣬ���ڵ���ƽ�⣺CH3COOH![]() CH3COO����H����ϡ�ʹٽ����룬��A�������ӵ����ʵ������ӣ���c(H��)���ͣ�A����B��ϡ�����������ӵ����ʵ������ӣ���������ʵ������٣����ֵ
CH3COO����H����ϡ�ʹٽ����룬��A�������ӵ����ʵ������ӣ���c(H��)���ͣ�A����B��ϡ�����������ӵ����ʵ������ӣ���������ʵ������٣����ֵ ����B��ȷ��C���¶Ȳ��䣬ˮ�����ӻ��������䣬��c(H��)��c(OH��)���䣬C����ѡB��
����B��ȷ��C���¶Ȳ��䣬ˮ�����ӻ��������䣬��c(H��)��c(OH��)���䣬C����ѡB��

 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�