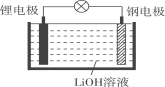
��Ŀ����
����Ŀ��ijѧ����0.2000mol��L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ��������£�
![]()
�� ������ˮϴ�Ӽ�ʽ�ζ��ܣ�������ע��NaOH��Һ����0���̶������ϣ�
�� �̶��õζ��ܲ�ʹ�ζ��ܼ������Һ�壻
�� ����Һ������0����0���̶������£������¶�����
�� ��ȡ20.00mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ��
�� �ñ�Һ�ζ����յ㣬���µζ���Һ�������
��ش�
��1�����ϲ���������ǣ����ţ�____�����ⶨ���ƫ�ߣ���ԭ�������______��
A�����Ʊ���Һ�Ĺ���NaOH�л���KOH����
B���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�����������ȷ
C��ʢװδ֪Һ����ƿ������ˮϴ��������δ֪Һ��ϴ
D���ζ����յ����ʱ�����ֵζ��ܼ��촦����һ����Һ
��2���жϵζ��յ��������____________________________��
��3������ͼ��ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ________mL��
��4�������������ݣ���������������Һ��Ũ��______mol/L��
�ζ����� | ���������mL�� | ���ռ������mL�� | |
�ζ�ǰ���� | �ζ������ | ||
��һ�� | 20.00 | 0.40 | 20.40 |
�ڶ��� | 20.00 | 2.00 | 24.10 |
������ | 20.00 | 4.00 | 24.00 |
���𰸡� �� AD �������һ������������Һ����Һ����ɫ��Ϊdz��ɫ���ұ���30�벻��ɫ 22.60 0.2000
����������1����ʽ�ζ���Ӧ������������ˮ��ϴ��Ȼ���ñ�NaOH��Һ��ϴ�������Ӱ���Һ��Ũ�ȣ��ʵ������������Ʊ���Һ�Ĺ���NaOH�л���KOH����������Һ�����������ӵ�Ũ��ƫС,���ĵ�V(��)������![]() ���ⶨ���ƫ��,����Aѡ������ȷ�����ζ��յ����ʱ,���ӵζ��ܵĿ̶�,���ĵ�V(��)ƫС����
���ⶨ���ƫ��,����Aѡ������ȷ�����ζ��յ����ʱ,���ӵζ��ܵĿ̶�,���ĵ�V(��)ƫС����![]() �������ⶨ���ƫ��������B������ʢװδ֪Һ����ƿ������ˮϴ��������δ֪Һ��ϴ�����ĵ�V(��)������
�������ⶨ���ƫ��������B������ʢװδ֪Һ����ƿ������ˮϴ��������δ֪Һ��ϴ�����ĵ�V(��)������![]() �������ⶨ�������,��C�������ζ����յ����ʱ�����ֵζ��ܼ��촦����һ����Һ�����ĵ�V(��)ƫ����
�������ⶨ�������,��C�������ζ����յ����ʱ�����ֵζ��ܼ��촦����һ����Һ�����ĵ�V(��)ƫ���� ![]() �������ⶨ���ƫ��������Dѡ������ȷ������ȷѡ��.AD��
�������ⶨ���ƫ��������Dѡ������ȷ������ȷѡ��.AD��
��2���ζ��յ�������������������һ��NaOH��Һʱ����ƿ����Һ����ɫ��Ϊdz��ɫ���ұ���30�벻��ʧ����ȷ�𰸣��������һ������������Һ����Һ����ɫ��Ϊdz��ɫ������30�벻��ʧ��
��3���ζ����е�Һ�����Ϊ![]() ����ȷ����22.60��
����ȷ����22.60��
��4�����εζ����ĵ����Ϊ20.00mL ��22.1 mL��20.00mL���ڶ������ݲ����������������������Ƶ����![]() ������
������![]() =0.2��20��10-3/20��10-3= 0.2000 mol/L����ȷ�𰸣�0.2000��
=0.2��20��10-3/20��10-3= 0.2000 mol/L����ȷ�𰸣�0.2000��

����Ŀ��Ϊ���������ܼ��š��͡���̼���á���Ŀǰ��ҵ����һ�ַ�������CO2������ȼ���Ҵ���һ�������·�����Ӧ��2CO2(g)��6H2(g)![]() CH3CH2OH(g)��3H2O(g)����H<0��
CH3CH2OH(g)��3H2O(g)����H<0��
��1����һ�������£���20 L�ܱ������а����ʵ�����Ϊ1��3����CO2��H2���¶���450 K��n(H2)��ʱ��仯�����ʾ��
t/min | 0 | 1 | 3 | 5 |
n(H2)/mol | 8 | 6 | 5 | 5 |
��450 �桢0��1 min��v(CH3CH2OH)��________�����¶��¸÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ________(���������λ��Ч����)��
��2����5 MPa�²��ƽ����ϵ�и����ʵ�����������¶ȵı仯������ͼ��ʾ��

�����ұ�ʾ���� ________(�����ʵĻ�ѧʽ)�����������ͼ����A���Ӧ���������b��________%(���������λ��Ч����)��
��3�����д�ʩ����ʹ��ѧƽ��������Ӧ�����ƶ�����________��
A�������¶�
B����CH3CH2OH(g)��ʱҺ�����
C��ѡ���Ч����
D���ٳ���l mol CO2��3 mol H2
��4��25 �桢1.01��105Paʱ��9.2 gҺ̬�Ҵ���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�273.4 kJ��������д����ʾ�Ҵ�ȼ�յ��Ȼ�ѧ����ʽ��________________________��
��5����ʯīΪ�缫���������ơ��Ҵ���ˮ������Ϊԭ�ϣ������Ƴ��Ҵ���ȼ�ϵ�أ�д��������ԭ��Ӧ�ĵ缫��Ӧʽ��_____________________________________��