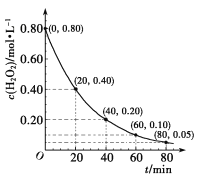
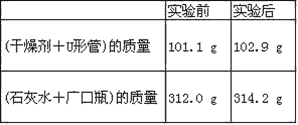
��Ŀ����
����Ŀ����֪��ѧ��Ӧ2C(s)��O2(g)![]() 2CO(g)��2CO(g)��O2(g)
2CO(g)��2CO(g)��O2(g)![]() 2CO2(g)���Ƿ��ȷ�Ӧ���ݴ��ƶ�����ͬ�����£�����˵������ȷ����(����)
2CO2(g)���Ƿ��ȷ�Ӧ���ݴ��ƶ�����ͬ�����£�����˵������ȷ����(����)
A.56 g CO��32 g O2�����е�������֮�ʹ���88 g CO2�����е�������
B.12 g C�����е�����һ������28 g CO�����е�����
C.12 g C��32 g O2���������������44 g CO2�����������
D.������������ȵ�̼��ȫȼ�գ�����CO2�ķ�Ӧ������CO�ķ�Ӧ�ų���������
���𰸡�B
��������
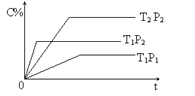
���ݷ��ȷ�Ӧ�з�Ӧ��������������������������������������ȫȼ��ʱ�ų����������ڲ���ȫȼ��ʱ���������ݴ������
A. ��2CO(g)+O2(g)![]() 2CO2(g)�Ƿ��ȷ�Ӧ������56 gCO��32 gO2�����е�����������88 gCO2�����е�������������Ӧ����������������������������A��ȷ��
2CO2(g)�Ƿ��ȷ�Ӧ������56 gCO��32 gO2�����е�����������88 gCO2�����е�������������Ӧ����������������������������A��ȷ��
B. ��2C(s)+O2(g)![]() 2CO(g)�Ƿ��ȷ�Ӧ������12 g C��16 g O2�����е�������һ������28 g CO�����е�������������12g C�����е���������28 g CO�����е�������B����
2CO(g)�Ƿ��ȷ�Ӧ������12 g C��16 g O2�����е�������һ������28 g CO�����е�������������12g C�����е���������28 g CO�����е�������B����
C. ��2C(s)+O2(g)![]() 2CO(g)�� 2CO(g)��O2(g)
2CO(g)�� 2CO(g)��O2(g)![]() 2CO2(g)���Ƿ��ȷ�Ӧ������C+O2
2CO2(g)���Ƿ��ȷ�Ӧ������C+O2![]() CO2Ҳ�Ƿ��ȷ�Ӧ������12 g C��32 g O2�����е�������һ������44 g CO2�����е���������C��ȷ��
CO2Ҳ�Ƿ��ȷ�Ӧ������12 g C��32 g O2�����е�������һ������44 g CO2�����е���������C��ȷ��
D. ��������ȫȼ�շų��������Ȳ���ȫȼ�շų������࣬����һ��������̼ȼ�գ�����CO2������COʱ�ų��������࣬D��ȷ��
�ʺ���ѡ����B��