��Ŀ����
��֪����101 kPaʱ��2C(s)��O2(g)=2CO(g)����H����221 kJ/mol����ϡ��Һ�У�H��(aq)��OH��(aq)=H2O(l)����H����57.3 kJ/mol�����н�����ȷ����(����)
| A��̼��ȼ���ȴ���110.5 kJ/mol |
| B���ٵķ�Ӧ��Ϊ221 kJ/mol |
| C��Ũ������ϡNaOH��Һ��Ӧ���к���Ϊ��57.3 kJ/mol |
| D��ϡ������ϡNaOH��Һ��Ӧ����1 molˮ���ų�57.3 kJ���� |
A
����

����˵����ȷ���ǣ� ��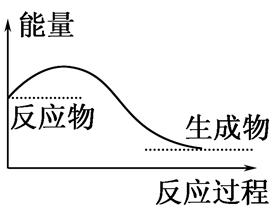
| A���κλ�ѧ��Ӧ�������������ı仯 |
| B��H2O��g���D��H2O��l���Ĺ��̷ų��������ȣ����Ըù����ǻ�ѧ�仯 |
| C����ѧ��Ӧ�������ı仯������Ϊ�����ı仯 |
| D����ͼ��ʾ�������������Ĺ��� |
��֪���� 2H2(g)��O2(g)=2H2O(g)����H����483.6 kJ��mol��1����H2(g)��S(g)=H2S(g)����H����20.1 kJ��mol��1��
�����ж���ȷ���ǣ� ��
| A��1 mol������ȫȼ������Һ̬ˮ��������241.8 kJ |
| B��1 mol H2O(g)��1 mol H2S(g)���������221.7 kJ |
| C���ɢ٢�֪��ˮ�����ȶ���С������ |
| D������Ӧ���и��ù�̬��1 mol S(s)��ȫ��Ӧ���ų�������С��20.1 kJ |
�����뻯ѧ��Ӧ�����仯��ص�������ȷ���� ����������
| A��������������һ�����ڷ�Ӧ�������� |
| B���ƾ�������ȼ�ϣ�˵���ƾ�ȼ�����ͷ������ķ�Ӧ |
| C���ɱ�������Ҫ���մ������ȣ�����仯�����ȷ�Ӧ |
| D��ͬ��ͬѹ�£�H2��g����Cl2��g��=2HCl��g���ڹ��պ͵�ȼ�����µĦ�H��ͬ |
FeCl3(aq)��KSCN(aq)���ʱ��������ƽ��:Fe3+(aq)+SCN-(aq) Fe(SCN)2+(aq)����֪ƽ��ʱ�����ʵ���Ũ��c��Fe(SCN)2+�����¶�T�Ĺ�ϵ��ͼ��ʾ��������˵����ȷ����( )
Fe(SCN)2+(aq)����֪ƽ��ʱ�����ʵ���Ũ��c��Fe(SCN)2+�����¶�T�Ĺ�ϵ��ͼ��ʾ��������˵����ȷ����( )
| A��FeCl3(aq)��KSCN(aq)��Ӧ���Ȼ�ѧ����ʽΪ:Fe3+(aq)+SCN-(aq)=Fe(SCN)2+(aq) ��H��0 |
| B���¶�ΪT1��T2ʱ����Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2����K1��K2 |
| C����Ӧ����D��ʱ��һ����v(��)��v(��) |
| D��A����B����ȣ�A���c(Fe3+)�� |
��ҵ������������У�SO2�ڽӴ����б�������ΪSO3����֪�÷�ӦΪ���ȷ�Ӧ���ֽ�2 mol SO2��1 mol O2����һ�ܱ�������ַ�Ӧ�ų�����98.3 kJ����ʱ���SO2��ת����Ϊ50%�������Ȼ�ѧ����ʽ��ȷ���� ����������
A��2SO3��g��  2SO2��g����O2��g����H����196.6 kJ��mol��1 2SO2��g����O2��g����H����196.6 kJ��mol��1 |
B��2SO2��g����O2��g��  SO3��g����H����98.3 kJ��mol��1 SO3��g����H����98.3 kJ��mol��1 |
C��SO2��g����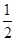 O2��g�� O2��g�� SO3��g����H����98.3 kJ��mol��1 SO3��g����H����98.3 kJ��mol��1 |
D��SO2��g����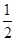 O2��g�� O2��g�� SO3��g����H����196.6 kJ��mol��1 SO3��g����H����196.6 kJ��mol��1 |
��֪:H2(g)+ O2(g)
O2(g) H2O(l)��H="-285.8" kJ/mol
H2O(l)��H="-285.8" kJ/mol
CH4(g)+2O2(g) CO2(g)+2H2O(l)��H="-890.3" kJ/mol
CO2(g)+2H2O(l)��H="-890.3" kJ/mol
����H2��CH4�Ļ������112 L(��״��),ʹ����ȫȼ������CO2(g)��H2O(l),���ų�����3 242.5 kJ,��ԭ���������H2��CH4�����ʵ���֮����(����)
| A��1��1 | B��1��3 | C��1��4 | D��2��3 |
�����й��������жϻ��ʾ������ȷ����(����)
| A����C(s��ʯī)=C(s�����ʯ)����H����1.9 kJ��mol��1����֪��ʯī�Ƚ��ʯ���ȶ� |
| B�����������������������ֱ���ȫȼ�գ����߷ų����������� |
| C����H��(aq)��OH��(aq)=H2O(l)����H����57.3 kJ��mol��1����֪����1 mol CH3COOH����Һ�뺬1 mol NaOH����Һ��ϣ��ų�����������57.3 kJ |
| D��2 g H2��ȫȼ������Һ̬ˮ�ų�285.8 kJ������������ȼ�յ��Ȼ�ѧ����ʽΪ2H2(g)��O2(g)=2H2O(l) |
����˵�����ʾ������ȷ���� (����)��
| A����Ӧ��������������������������ʱ����Ӧһ�������Է����� |
| B����֪��H2S(g)��aO2(g)=x��bH2O(l)����H������H��ʾH2S��ȼ���ȣ���xΪSO2(g) |
| C����֪��2SO2(g)��O2(g)??2SO3(g)����H����98.3 kJ��mol��1�����ܱ������г���1 mol SO2��0.5 mol O2����ַ�Ӧ��ų�49.15 kJ������ |
| D����ʯī�Ƚ��ʯ�ȶ��ɵã�C(s�����ʯ)=C(s��ʯī)����H>0 |