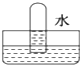
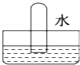
��Ŀ����
7�� ���ᣨ�Ҷ��ᣩ������ԭ���ͳ����������ڽ������⡢֯��Ư��ϡ��������һ���Ʊ����ᾧ�壨H2C2O4•2H2O���Ĺ���������ͼ��
���ᣨ�Ҷ��ᣩ������ԭ���ͳ����������ڽ������⡢֯��Ư��ϡ��������һ���Ʊ����ᾧ�壨H2C2O4•2H2O���Ĺ���������ͼ����1��д���ڢڲ�����Ļ�ѧ��Ӧ����ʽ2HCOONa$\frac{\underline{\;\;��\;\;}}{\;}$Na2C2O4+H2����
��2���û�ѧʽ��ʾ��ACaC2O4������CaSO4��
��3�����֤���ڢٲ���Ӧ�м��������ɣ�ʵ�����Ϊȡ�ٷ�Ӧ�����Һ���Թ��У�����������Һ����������������������ټ������ᣬ���ɵ�����ͨ������ʯ��ˮ����ǣ�����֤���м��������ɣ�
��4�����˽�������������ֱ���������ữ�Ʊ����ᣮ�÷�����ȱ���Dz�Ʒ���������к��е�������Ҫ��Na2SO4��
��5���ڢ۲��õ��IJ��ᾧ��Ĵ����ø�����ط��ⶨ�����������Ʒ0.250g����ˮ����0.0500mol•L-1������KMnO4��Һ�ζ�����dz�ۺ�ɫ������ڲ����ʣ�����KMNO4��Һ15.00mL����ò����Ʒ�Ĵ���Ϊ94.5%��������ʵ������в�����KMnO4��Һ����1������ƿ�⣬������������ƫ�ߣ��ƫ�ߡ�����ƫ�͡�����Ӱ�족��
���� ��������ͼ֪��һ�������£�һ����̼���������Ʒ�Ӧ���ɼ����ƣ���Ӧ����ʽΪ��CO+NaOH$\frac{\underline{\;һ������\;}}{\;}$HCOONa�����������£��������������ɲ����ƣ���ԭ���غ��֪���������ɣ���Ӧ����ʽΪ��2HCOONa$\frac{\underline{\;\;��\;\;}}{\;}$Na2C2O4+H2�������������������Ʒ�Ӧ�õ���������������ƣ���Һ��NaOH��Һ��A��CaC2O4������������ᷴӦ�õ������������ƣ�������ΪCaSO4����ҺBΪ��������Һ���پ����ᾧ������õ������ƾ��壮
��3������������Ӧ�������������Ƿ���ȩ��������������Ӧ����Һ�м���������ữ���ٽ�����ͨ������ʯ��ˮ�����ж�����̼���ɣ�����֤���м��������ɣ�
��4���������������Ͽɵõ����ἰ�����ƣ�
��5��������Ӧ��5H2C2O4+2MnO4-+6H+=10CO2��+2Mn2++8H2O�����ݷ���ʽ������Ʒ�в��������������������������������������ʵ������в�����KMnO4��Һ����1������ƿ�⣬�������ĸ��������Һ�����ƫ����ⶨ���������ƫ��
��� �⣺��������ͼ֪��һ�������£�һ����̼���������Ʒ�Ӧ���ɼ����ƣ���Ӧ����ʽΪ��CO+NaOH$\frac{\underline{\;һ������\;}}{\;}$HCOONa�����������£��������������ɲ����ƣ���ԭ���غ��֪���������ɣ���Ӧ����ʽΪ��2HCOONa$\frac{\underline{\;\;��\;\;}}{\;}$Na2C2O4+H2�������������������Ʒ�Ӧ�õ���������������ƣ���Һ��NaOH��Һ��A��CaC2O4������������ᷴӦ�õ������������ƣ�������ΪCaSO4����ҺBΪ��������Һ���پ����ᾧ������õ������ƾ��壮
��1��������������֪���ڢڲ�����Ļ�ѧ��Ӧ����ʽΪ��2HCOONa$\frac{\underline{\;\;��\;\;}}{\;}$Na2C2O4+H2����
�ʴ�Ϊ��2HCOONa$\frac{\underline{\;\;��\;\;}}{\;}$Na2C2O4+H2����
��2��������������֪��AΪCaC2O4������ΪCaSO4��
�ʴ�Ϊ��CaC2O4��CaSO4��
��3��֤���м��������ɵ�ʵ�鷽��Ϊ��ȡ�ٷ�Ӧ�����Һ�����Թ��У�����������Һ����������������������ټ������ᣬ���ɵ�����ͨ������ʯ��ˮ����ǣ�����֤���м��������ɣ�
�ʴ�Ϊ��ȡ�ٷ�Ӧ�����Һ���Թ��У�����������Һ����������������������ټ������ᣬ���ɵ�����ͨ������ʯ��ˮ����ǣ�����֤���м��������ɣ�
��4�������������IJ���Ϊ�����ƣ�ֱ���������ữ�����ɲ���������ƣ����к��е�������Ҫ��Na2SO4���ʴ�Ϊ��Na2SO4��
��5���ڲⶨ�����У��������Ϊ������������Ϊ��ԭ������Ӧ�����ӷ���ʽΪ��5H2C2O4+2MnO4-+6H+=10CO2��+2Mn2++8H2O�����ݷ���ʽ�ɵù�ϵʽ��
5H2C2O4•2H2O��2KMnO4
5 2
n 0.05mol/L��15.0��10-3L
���n��H2C2O4•2H2O��=1.875��10-3mol
��m��H2C2O4•2H2O��=1.875��10-3mol��126g/mol=0.236g
���Գ�Ʒ�Ĵ��Ȧ�=$\frac{0.236g}{0.25g}$��100%=94.5%��
������ʵ������в�����KMnO4��Һ����1������ƿ�⣬�������ĸ��������Һ�����ƫ����ⶨ���������ƫ�ʲ�ò������������ƫ�ߣ�
�ʴ�Ϊ��94.5%��ƫ�ߣ�
���� ���⿼��ʵ���Ʊ���������ѧ�뼼���������������������̡�������ԭ��Ӧ�ζ����㡢���ʺ����IJⶨ�ȣ���ȷԭ���ǽ���ؼ����Ƕ�ѧ���ۺ������Ŀ��飬�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | �������뻹ԭ�������ʵ���֮��Ϊ2��1 | |
| B�� | ���������뻹ԭ��������ʵ���֮��Ϊ1��1 | |
| C�� | �÷�Ӧ˵��Cu�Ľ����Ժ�ǿ | |
| D�� | 2mol H2SO4���뷴Ӧʱ����4 mol���ӷ���ת�� |
| A�� | -OH��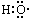 ����ʾ�ǻ� ����ʾ�ǻ� | B�� | �۱�ϩ�Ľṹ��ʽ��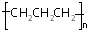 | ||
| C�� | CH4���ӵı���ģ�ͣ� | D�� | ��������ӵĵ���ʽ��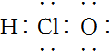 |
| A�� | HBr��HCl��HF | B�� | HF��H2O��NH3 | C�� | NH3��PH3��H2S | D�� | SiH4��CH4��NH3 |
| A�� | ԭ��������W��X��Y��Z | |
| B�� | ԭ�Ӱ뾶��r��W����r��X����r��Y����r��Z�� | |
| C�� | ����������Ӧ��ˮ������ԣ�w��x | |
| D�� | �ڵ������У�W3XZ6����Ҫ��������ǿ����ʵĵ����� |
| A�� | 1.6g�������ͳ�����ɵĻ�����к�����ԭ�ӵ���ĿΪ0.1NA | |
| B�� | 18gD2O����10NA������ | |
| C�� | ��״���£�2.24L CCl4�к���C-Cl 0.4 NA | |
| D�� | ��״���£�0.1 mol Cl2 ����ˮ��ת�Ƶĵ�����ĿΪ0.1NA |