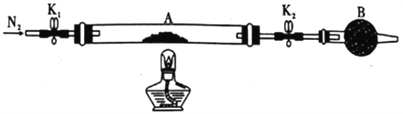
��Ŀ����
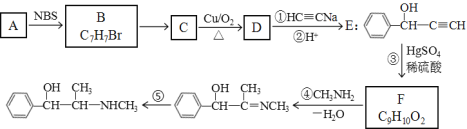
����Ŀ��A��B��C��D��E��F����ѧ��ѧ�г��������ֶ�����Ԫ�أ��й�λ�ü���Ϣ���£�A������������Ӧ��ˮ���������⻯�ﷴӦ�������ӻ����C����һ�㱣����ú���У�F������������Ӧ��ˮ���������ǿ�ᷴӦ������ǿ�Ӧ��G�������ճ��������������Ľ������ױ���ʴ������ش��������⣺
��1��AԪ�ص��⻯��ˮ��Һ��ʹ��̪����ԭ���õ��뷽��ʽ����Ϊ_____________��
��2��ͬ��ͬѹ�£���a L A�⻯��������b L D���⻯�������Ⱥ�ͨ��һʢˮ���ձ��У���������Һ��pH=7����a_________b(����>"����<������=��)��
��3�������£���ͬŨ��F��G�����ӵ���Һ�еμ�NaOH��Һ��F��G��Ԫ���Ⱥ������F(OH)n��ȫ������pH��4.7��G(OH)n��ȫ������pH��2.8��������ͬ�����£��ܽ�Ƚϴ���ǣ�___���ѧʽ����
��4��A��B�����������Ϊ7:16����ԭ�ӷ��ӣ��÷����ͷ��ڿ������仯ѧ���ÿ��������ĺ���У�_______________��
������ ������ЧӦ �۹⻯ѧ���� �ܳ������ƻ�
��5��A��C��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּ�÷�Ӧ�Ļ�ѧ����ʽ��______��
��6���õ���ʽ��ʾB,C�γɻ�����C2B2�Ĺ���_______________��
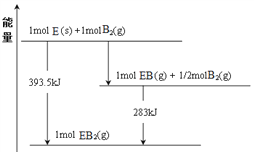
��7����֪һ������E��������B2(g)��ȼ�գ�����ܵIJ��P������ϵ����ͼ��ʾ����д��һ��������EB2(g) ��E(s)��Ӧ����EB(g)���Ȼ�ѧ����ʽ__________________��
���𰸡� NH3��H2O![]() NH4+ + OH�� �� Al(OH)3 �٢� Na3N + 4H2O��3NaOH + NH3��H2O
NH4+ + OH�� �� Al(OH)3 �٢� Na3N + 4H2O��3NaOH + NH3��H2O ![]() CO2(g) + C(s) = 2CO(g) ;��H= +172.5kJ/mol
CO2(g) + C(s) = 2CO(g) ;��H= +172.5kJ/mol
��������A������������Ӧ��ˮ���������⻯�ﷴӦ�������ӻ����A�ǵ�Ԫ�أ�C����һ�㱣����ú���У�C����Ԫ�أ�F������������Ӧ��ˮ����������ᷴӦ������Ӧ��F����Ԫ�أ�G�������ճ��������������Ľ������ױ���ʴ����G����Ԫ�أ����ݱ���B����Ԫ�أ�E��̼Ԫ�أ�D����Ԫ�ء���1��AԪ�ص��⻯����NH3������ˮ�õ�����Һ��ˮ�������ԣ��ܵ������������ʹ��̪��죺NH3��H2O![]() NH4+ + OH������2��ͬ��ͬѹ�£���a L NH3�����b L HCl����ͨ��ˮ�У������Ȼ�泥�ǿ�������Σ���Һ�����ԣ���������Һ��pH=7������Ҫ��������a>b����3�������£���ͬŨ�ȣ���Ũ�Ⱦ�Ϊc��Al��Fe�����ӵ���Һ�еμ�NaOH��Һ��Al(OH)3��ȫ������pH��4.7����Ksp=c��c3(OH-)=(10-9.3)3��c=10-27.9��c��Fe(OH)3��ȫ������pH��2.8����Ksp= c��c3(OH-)= (10-11.2)3��c=10-33.6��c����ksp�ϴ����Al(OH)3����4��A��B���������Ϊ7:16����ԭ�ӷ�����NO2��������ˮ���ͷ��ڿ����п�����������⻯ѧ������������ЧӦ����Ϊ������̼���ŷţ��������ƻ��Ƿ���������ɵġ���ѡ�٢ۣ���5��N��Na��ɵ����ӻ�������Na3N������ˮ��Ӧ�������ּ�÷�Ӧ�Ļ�ѧ����ʽ��Na3N + 4H2O��3NaOH + NH3��H2O����6���õ���ʽ��ʾO��Na�γɻ�����Na2O2�Ĺ���Ϊ��
NH4+ + OH������2��ͬ��ͬѹ�£���a L NH3�����b L HCl����ͨ��ˮ�У������Ȼ�泥�ǿ�������Σ���Һ�����ԣ���������Һ��pH=7������Ҫ��������a>b����3�������£���ͬŨ�ȣ���Ũ�Ⱦ�Ϊc��Al��Fe�����ӵ���Һ�еμ�NaOH��Һ��Al(OH)3��ȫ������pH��4.7����Ksp=c��c3(OH-)=(10-9.3)3��c=10-27.9��c��Fe(OH)3��ȫ������pH��2.8����Ksp= c��c3(OH-)= (10-11.2)3��c=10-33.6��c����ksp�ϴ����Al(OH)3����4��A��B���������Ϊ7:16����ԭ�ӷ�����NO2��������ˮ���ͷ��ڿ����п�����������⻯ѧ������������ЧӦ����Ϊ������̼���ŷţ��������ƻ��Ƿ���������ɵġ���ѡ�٢ۣ���5��N��Na��ɵ����ӻ�������Na3N������ˮ��Ӧ�������ּ�÷�Ӧ�Ļ�ѧ����ʽ��Na3N + 4H2O��3NaOH + NH3��H2O����6���õ���ʽ��ʾO��Na�γɻ�����Na2O2�Ĺ���Ϊ��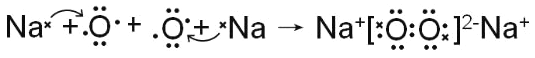 ����6����������ͼ����C(s)+O2(g)=CO2(g) ��H1=-393.5kJ/mol����CO(g)+
����6����������ͼ����C(s)+O2(g)=CO2(g) ��H1=-393.5kJ/mol����CO(g)+ ![]() O2(g)=CO2(g) ��H2=-283.0kJ/mol�����ݸ�˹�����ɢ�-2���ڵã�CO2(g) + C(s) = 2CO(g) ��H= +172.5kJ/mol��
O2(g)=CO2(g) ��H2=-283.0kJ/mol�����ݸ�˹�����ɢ�-2���ڵã�CO2(g) + C(s) = 2CO(g) ��H= +172.5kJ/mol��

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�����Ŀ����ҵ����﮻�ʯΪԭ������̼��﮵IJ��ֹ�ҵ�������£�
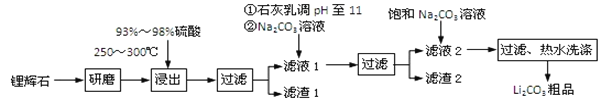
��֪��
��﮻�ʯ����Ҫ�ɷ�ΪLi2O��Al2O3��4SiO2�����к�����Ca��MgԪ�ء�
��Li2O��Al2O3��4SiO2 + H2SO4��Ũ�� ![]() Li2SO4 + Al2O3��4SiO2��H2O
Li2SO4 + Al2O3��4SiO2��H2O
��ijЩ���ʵ��ܽ�ȣ�s�����±���ʾ��
T/�� | 20 | 40 | 60 | 80 |
s��Li2CO3��/g | 1.33 | 1.17 | 1.01 | 0.85 |
s��Li2SO4��/g | 34.2 | 32.8 | 31.9 | 30.7 |
��1�����������з����Al2O3����������ͼ��ʾ����д�����ɳ��������ӷ���ʽ______��
![]()
��2����֪����2����Ҫ�ɷ���Mg��OH��2��CaCO3������Һ1�м���ʯ����������ǣ����û�ѧƽ��ԭ��������________________________________________________��
��3�����һ�������У�������ˮϴ������ԭ����______________________________��
��4����ҵ�ϣ���Li2CO3��Ʒ�Ʊ��ɸߴ�Li2CO3�IJ��ֹ������£�
a.��Li2CO3�������������۵�����Һ��LiOH��Һ������Һ������������ѡ����Ĥ�������ö��Ե缫��⡣
b.������LiOH��Һ�м�������NH4HCO3��Һ�����ȣ����ˡ���ɵøߴ�Li2CO3��
��a�У������ĵ缫��Ӧʽ��_________________________
�ڵ���LiOH��ҺŨ�������ԭ��_________________��b������Li2CO3��Ӧ�Ļ�ѧ����ʽ��___________________________________________��
��5����������﮵���ܷ�ӦΪ��FePO4+Li![]() LiFePO4������еĹ������ʿɴ���Li+����д���õ�طŵ�ʱ��������Ӧ��__________________��
LiFePO4������еĹ������ʿɴ���Li+����д���õ�طŵ�ʱ��������Ӧ��__________________��