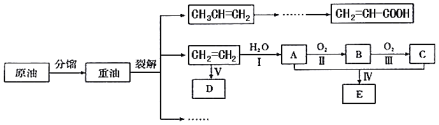
��Ŀ����
����Ŀ���������Ƴ�����Ư����ɱ������������������ˮ�Ͷ�����̼�����ʷ�����Ӧ�����治��ʱ���ױ��ʡ�ijʵ��С���Թ�������Ϊ�о��������������ʵ�顣
(1)̽��һ��Na2O2��Ʒ�Ƿ��Ѿ����ʣ�ȡ������Ʒ�������ܽ⣬����__________��Һ��������а�ɫ������֤��Na2O2�Ѿ����ʡ�
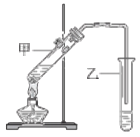
(2)��ʵ��С��Ϊ�˴��Բⶨ�������Ƶ�������������ȡ��m g��Ʒ�����������ͼװ�����ⶨ�������Ƶ�����������
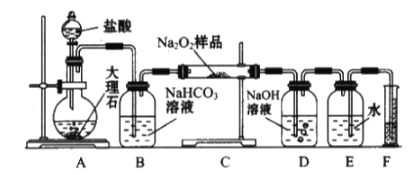
��װ�������ڵμ������������������_________��װ��D��������_________________________________��
�ڽ��������Ӻ��Ժ�����еĵ�һ��������_________________________________��
��д��װ��C�з�����Ҫ��Ӧ�Ļ�ѧ����ʽ_________________________________��
�ܷ�Ӧ�������ڶ�ȡʵ����������������ʱ������Ϊ��������_______________(�����)��
a����ȡ�������ǰ������ȴ������
b��������ͲʹE��F��Һ��߶���ͬ
e�������밼Һ�����͵���ƽʱ��ȡ��Ͳ��ˮ�����
�ݶ�����Ͳ��ˮ�������������ɱ�״�������������ΪVmL������Ʒ�й������Ƶ���������Ϊ______________________________��
���𰸡�BaCl2 ��Һ©�� ���������л��еĶ�����̼���� ���װ�������� 2Na2O2 + 2CO2 == 2Na2CO3 + O2 abc 39v/(56m)%
��������
��1�����������ڿ����б��ʻ��������̼���ƹ��壬����̼������Ӽ����Ƿ���ʣ�
��2��װ��ͼ��AΪ���ɶ�����̼��װ�ã�BΪϴ��װ�ã�CΪ������̼��������Ʒ�Ӧ��װ�ã�DΪ���ն���Ķ�����̼��װ�ã�E��F�Dz������������������װ�ã�
��װ�������ڵμ����������������Ϊ��Һ©����DΪ���ն���Ķ�����̼��װ�ã�
������װ��ͼ������֪���ⶨ�����Dzⶨ������̼�����ɷ�Ӧ���ɵ�������װ���б�������������ã�
��װ��C���Ƕ�����̼�������Ʒ�Ӧ����̼���ƺ������ķ�Ӧ
��������Ͳ��ȡҺ�����ʱ��Ҫ�ͼ���ƿҺ����ƽ���¶��ڳ����£�
��������������������������ʵ�������ϻ�ѧ����ʽ����õ������������ʵ������õ��������Ƶ�����������
��1�����������ڿ����б��ʻ��������̼���ƹ��壬̽��һ������������Ʒ�Ƿ��Ѿ����ʣ���������̼������Ӻͱ����ӽ������̼��Ӱ�ɫ����֤��Na2O2�Ѿ����ʣ�ȡ������Ʒ���ܽ⣬����BaCl2��Һ��������а�ɫ������֤��Na2O2�Ѿ����ʣ��ʱ����Ϊ�� BaCl2��
��1����װ�������ڵμ����������������Ϊ��Һ©���� DΪ���ն���Ķ�����̼��װ�ã���ֹ����Ķ�����̼��������װ�ã����²�õ����������ƫ�ʱ����Ϊ����Һ©�������������л��еĶ�����̼���壻
��ʵ��̽���ⶨ�����Dzⶨ������̼�������Ʒ�Ӧ���ɵ�������װ���б�������������ã����������Ӻ��Ժ�����еĵ�һ�������Ǽ��װ�õ������ԣ��ʱ����Ϊ�����װ�õ������ԣ�
��װ��C���Ƕ�����̼�������Ʒ�Ӧ����̼���ƺ������ķ�Ӧ����Ӧ����Ҫ��Ӧ�Ļ�ѧ����ʽΪ��2Na2O2 + 2CO2 == 2Na2CO3 + O2���ʱ����Ϊ��2Na2O2 + 2CO2 == 2Na2CO3 + O2��
��a�ֱ�Ӷ�ȡ�������������ȴ�����£���ʹ��Һ��������������������˶�ȡ�������ǰ������ȴ�����£�a��ȷ��b�������Ͳ����Һ��߶�ʹ֮��ͬ��ʹװ����ѹǿ�����ѹǿ��ͬ�������ȡ���������b��ȷ��c������밼Һ�����͵���ƽʱ����ȡ��Ͳ��ˮ�������ȷ��c��ȷ���ʱ����Ϊ��abc��
�ݲⶨ����Ͳ��ˮ�����������ɱ�״�������������ΪVmL�����ʵ���Ϊ��![]() ������Ʒ�й������Ƶ���������Ϊ��
������Ʒ�й������Ƶ���������Ϊ��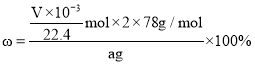 ���
���![]() ���ʱ����Ϊ��
���ʱ����Ϊ��![]() ��
��