��Ŀ����
����Ŀ�������ǻ����л��ϳ�ԭ�ϣ��㷺����ҽҩ��ұ��ͻ����Ȳ��š�ijУ����С���ͬѧ��������Ȳ�ϳ�H2C2O4��2H2O���ش���������:
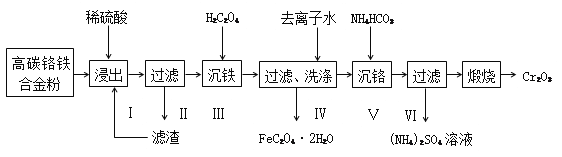
��1�������ͬѧ���õ�ʯ����Ҫ�ɷ�CaC2����CaS��Ca3P2�����ʣ���ȡC2H2[��Ӧ����ʽΪCaC2 +2H2O=Ca(OH)2 +C2H2(g) ��H��0���÷�Ӧ����]��ʵ��װ�����£�

A B C
��װ��A�У�Ϊ��С������Ȳ�����ʣ����ñ���ʳ��ˮ����ˮ�⣬���ɲ�ȡ�Ĵ�ʩ��_____����һ������
��װ��A�У�Ca3P2��ˮ��Ӧ�Ļ�ѧ����ʽΪ_______________________________________��
װ��B�У�NaClO����ԭΪ�Ȼ��ƣ���Ӧ�����л��м�����Cl2���ɡ�д��H2S��NaClO��Һ����Ϊ�������Ҫ��Ӧ�����ӷ���ʽ:___________________________��
��װ�DC��NaOH��Һ��������___________________________________________________��
��2�������ͬѧ���ü�����ȡ����Ȳ��Ũ������Hg(NO3)2���·�Ӧ�������ᾧ���ؽᾧ��H2C2O4��2H2O���Ʊ�װ������:
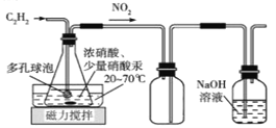
D E F
��װ��D�ж�����ݵ�������___________________________________________��װ��E��������__________________________________________________________��
��װ��D����H2C2O4 ��Ӧ�Ļ�ѧ����ʽΪ______________________________________��
��3�������ͬѧ�����ʵ��֤��������л�ԭ�ԡ���ʵ�鷽��Ϊ_____________��
���𰸡� �����μӱ���ʳ��ˮ������ƿ���ڱ�ˮ�У� Ca3P2+6H2O===3Ca(OH)2 +PH3�� H2S+4ClO- ===SO42- +4Cl- +2H+ ����Cl2���������� ������Ȳ����������ĽӴ��� ��ֹ����  ȡ����������Һ���Թ��У�����KMnO4��Һ����Һ��ɫ��ȥ
ȡ����������Һ���Թ��У�����KMnO4��Һ����Һ��ɫ��ȥ
����������1��������Ϣ��֪���÷�Ӧ���ң���Ҫ������Ӧ���ʣ����ñ���ʳ��ˮ����ˮ�⣬�����Խ���ƿ���ڱ�ˮ�н����¶ȣ���ȻҲ���Կ��Ƶμӱ���ʳ��ˮ���ٶȡ���Ca3P2��ˮ��Ӧ����PH3�����Ca(OH)2����ѧ����ʽΪ��Ca3P2+6H2O=3Ca(OH)2+PH3����H2S��NaClO��Һ����Ϊ������ClO-����ԭΪCl-����Ӧ�����ӷ���ʽΪ��H2S+4ClO-=SO42-+4Cl-+2H+������Ϊ��Ӧ�����л���Cl2���ɣ���������ʣ���H2S��PH3���������壬����װ�DC��NaOH��Һ��������������Cl2������������
��2���ٶ�����ݿ�������������Һ���ĽӴ������ʹ��Ȳ�������ַ�Ӧ��������������Ϊ�˷�ֹ��������D��F֮���װ��E����ȫƿ���ڸ�����֪����ͼ��ʾ����Ӧ��ΪC2H2��HNO3��������ΪNO2��H2C2O4�����ݵ�ʧ�����غ㼰ԭ���غ㣬װ��D����H2C2O4 ��Ӧ�Ļ�ѧ����ʽΪ��C2H2+8HNO3![]() H2C2O4+8NO2+4H2O��
H2C2O4+8NO2+4H2O��
��3��Ҫ֤��������л�ԭ�ԣ���ѡ����������֮��Ӧ�����磺����FeCl3��Һ����������Һ�������Һ��Ӧ���۲���ɫ�仯��

 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�����Ŀ����һ����ɱ���ܱ������У�����һ������X��Y��������ӦmX��g��![]() nY��g������H =QkJ/mol����Ӧ�ﵽƽ��ʱ��Y�����ʵ���Ũ�����¶ȡ���������Ĺ�ϵ���±���ʾ��
nY��g������H =QkJ/mol����Ӧ�ﵽƽ��ʱ��Y�����ʵ���Ũ�����¶ȡ���������Ĺ�ϵ���±���ʾ��
| 1 | 2 | 3 |
100 | 1.00 | 0.75 | 0.53 |
200 | 1.20 | 0.09 | 0.63 |
300 | 1.30 | 1.00 | 0.70 |
����˵����ȷ����
A.m��n B. �¶Ȳ��䣬ѹǿ����Y��������������
C. Q��0 D.������䣬�¶����ߣ�ƽ�����淴Ӧ�����ƶ�