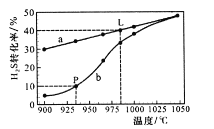
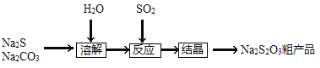
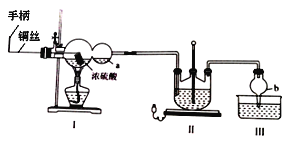
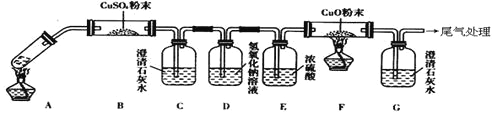
��Ŀ����
����Ŀ��Ϊ̽�����������ķֽ�������������װ����ͼ��ʾ��װ��a�У���K1��K2,����ͨ��N2�����ȡ�ʵ���Ӧ���в�������Ϊ��ɫ��ĩ������˵������ȷ����
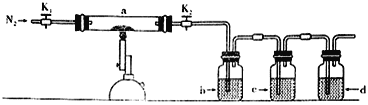
A. �ֽ�������������SO3��SO2,Ӧ���ȼ���SO3
B. װ��b�е��Լ�ΪBa(NO3)2��Һ����Ӧ���а�ɫ��������
C. װ��c�е��Լ�Ϊ����KMnO4��Һ,�����dz�ȥ��������е�SO2
D. װ��d�е��Լ�Ϊʯ��ˮ����β������װ��
���𰸡�A
��������
A.�������������·ֽ�������������������������������Ȱѻ������ͨ���Ȼ�����Һ�У������������Ȼ�����Һ����Ӧ�����������������Ȼ�����Һ��Ӧ�������ᱵ��ɫ��������˷ֽ�����������Ӧ���ȼ���SO3����A��ȷ��
B.����Ѷ����������壬ͨ�����ᱵ��Һ�У���Ϊ������������ˮ��Һ�����ԣ����������£���������ӽ�������������Ϊ��������ӣ����뱵�����������ᱵ���������������ᱵ��Һ�������ǣ����ж�ʹ���ᱵ��Һ���ְ�ɫ�������Ƕ�����������������B����
C.װ��c�е��Լ�Ϊ����KMnO4��Һ,�����Ǽ����������е�SO2�������Һ��ɫ��֤������SO2����C����
D.װ��d��װӦ��������������Һ�����ն���Ķ���������������D������
��ѡA��

����Ŀ��ǰ������Ԫ�� R��X��Y��Z��E��ԭ�������������ӣ����ǵĽṹ�Ͳ�����Ϣ���±���ʾ��
Ԫ�ش��� | ������Ϣ |
R | ��̬Rԭ�Ӻ����������ܼ���ÿ���ܼ��ϵ�������ͬ |
X | X��˫ԭ�ӵ��ʦļ��ͦм���Ŀ֮��Ϊ1��2 |
Y | ����������Ԫ���У�ԭ�Ӱ뾶��� |
Z | Z����������ϼ���������ϼ�֮�͵���4 |
E | ��̬E3+����Χ�����Ų�ʽ��3d5 |
�ش����⣺
��1��EԪ�������ڱ��е�λ����________�����̬ԭ���е���ռ�ݵ�����ܲ���__________________��
��2��Ԫ�� X���⻯��M�������ں�18�����ӣ�M�ĽṹʽΪ_____��ÿ������ԭ�ӵļ۲���Ӷ�����_________________��
��3����R��X��Z�ĺ�����������У���Ϊ�ȵ��������������_________________��
��4��ZԪ�ص������������Ӧ��ˮ�����У����Խ�ǿ��________����ԭ����_________________��
��5��(ZX)4�ڳ�ѹ�£�����130��ʱ(ZX)4�ֽ�Ϊ��Ӧ�ĵ��ʣ���һ�仯�ƻ�����������________________����Ϊ��ɫ�Թ��壬����ɫ��ЧӦ������-30��ʱΪ����ɫ������100��ʱΪ���ɫ.
�ڵ���ɫ���Ȼ�ɫ�����ɫ��ת���У��ƻ�����������_________________��
��6�����������£�E�ľ��������ͼ��ʾ�Ķѻ���ʽ�������ֶѻ�ģ�͵���λ��Ϊ__________����Eԭ�ӵİ뾶Ϊr������E��ԭ�ӿռ�������Ϊ________________�����г�����ʽ���ɣ�