��Ŀ����
����Ŀ���Լ״�Ϊԭ����ȡ�ߴ�H2����Ҫ���о����ش��������⣺
��1���״�ˮ��������������Ҫ��������������Ӧ��
����Ӧ��CH3OH(g)��H2O(g)![]() CO2(g)��3H2(g) ��H����49kJ��mol��1
CO2(g)��3H2(g) ��H����49kJ��mol��1
����Ӧ��H2(g)��CO2(g) ![]() CO(g)��H2O(g) ��H����41kJ��mol��1
CO(g)��H2O(g) ��H����41kJ��mol��1
�ټ״��ڴ����������ѽ�ɵõ�H2��CO����Ӧ���Ȼ�ѧ����ʽΪ__________________�����ܼӿ췴Ӧ�����������CH3OHƽ��ת���ʵ�һ�ִ�ʩ��_________________________��
���ʵ�����ˮ����[n(H2O)��n(CH3OH)]�������ڼ״�ˮ�����������⣬������___________��
��ij�¶��£���n(H2O)��n(CH3OH)��1��1��ԭ������������ܱ������У���ʼѹǿΪp1����Ӧ�ﵽƽ��ʱ��ѹǿΪp2����ƽ��ʱ�״���ת����Ϊ_________________�����Ը���Ӧ����
��2����ҵ�ϳ���CH4��ˮ������һ������������ȡH2���䷴Ӧԭ��Ϊ��CH4(g)��H2O(g)![]() CO(g)��3H2(g) ��H����203kJ��mol��1�����ݻ�Ϊ3L���ܱ�������ͨ�����ʵ�����Ϊ3mol��CH4��ˮ��������һ�������·���������Ӧ�����ƽ��ʱH2������������¶ȼ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
CO(g)��3H2(g) ��H����203kJ��mol��1�����ݻ�Ϊ3L���ܱ�������ͨ�����ʵ�����Ϊ3mol��CH4��ˮ��������һ�������·���������Ӧ�����ƽ��ʱH2������������¶ȼ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
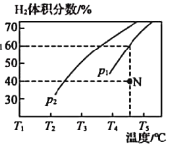
ѹǿΪp1ʱ����N�㣺v��_______v��(����ڡ���С�ڡ����ڡ�)��N���Ӧ�¶��¸÷�Ӧ��ƽ�ⳣ��K��________mol2��L��2��
���𰸡�CH3OH(g)CO(g)��2H2(g) ��H��+90kJ/mol �����¶� ��״���ת���ʣ�������������CO������ ��p2/p1-1����100% ���� 48
��������
��1�����ݸ�˹����ȷ���Ȼ�ѧ����ʽ������������ʽ���м��㣻
��2������ͼ��N�������ƶ���60%ʱ���ﵽƽ��״̬�������ƶ�������ƽ��ʱ�����ʵ�Ũ�ȸ��ݷ���ʽ����K��
��1���ٸ��ݸ�˹���ɣ�����Ӧ+����Ӧ���ɵõ�CH3OH(g)CO(g)��2H2(g) ��H��+90kJ/mol�����CH3OHƽ��ת������Ҫƽ�������ƶ�����ӦΪ���ȷ�Ӧ�������¶ȼ�����߷�Ӧ���ʣ��ֿ���״���ת���ʣ�
���ʵ�����ˮ����[n(H2O)��n(CH3OH)]����ʹ����Ӧ�����ƶ�������CO�����ɣ�����״���ת���ʣ�
�� CH3OH(g)��H2O(g)![]() CO2(g)��3H2(g)
CO2(g)��3H2(g)
��ʼ��1 1
��Ӧ��x x x 3x
ƽ�⣺1-x 1-x x 3x
�ں��¡������ܱ��У�ѹǿ֮�ȵ������ʵ���֮�ȣ�2����2+2x��=p1��p2�����x=p2/p1-1���״���ת����=x/1��100%=��p2/p1-1����100%��
��2������ͼ���֪��ѹǿΪp1ʱ���������������Ϊ60%ʱ����Ӧ�ﵽƽ��״̬�����������������ʵ�������ƽ�������ƶ�����v������v����ƽ��ʱ�������������Ϊ60%����
CH4(g)��H2O(g)![]() CO(g)��3H2(g)
CO(g)��3H2(g)
��ʼ��3 3
��Ӧ��x x x 3x
ƽ�⣺3-x 3-x x 3x
3x/��6+2x��=60%�����x=2��ƽ��ʱ�������ʵ�Ũ�ȷֱ�Ϊ1/3mol/L��1/3mol/L��2/3mol/L��2mol/L��K=2/3��23/��1/3��1/3��=48��

����Ŀ���¶�ΪTʱ���������ݻ���Ϊ1L�ĺ����ܱ������н�������Ӧ��2SO2(g)��O2(g)![]() 2SO3(g) ��H��0���ﵽƽ��ʱ������˵������ȷ����
2SO3(g) ��H��0���ﵽƽ��ʱ������˵������ȷ����
���� ��� | �������� | ��ʼ���ʵ��� / mol | ƽ��ʱSO3�����ʵ��� / mol | ||
SO2 | O2 | SO3 | |||
I | ���º��� | 2 | 1 | 0 | 1.8 |
II | ���º�ѹ | 2 | 1 | 0 | a |
III | ���Ⱥ��� | 0 | 0 | 2 | b |
A. ����I��SO2��ת����С������II��SO2��ת����
B. ����II��ƽ�ⳣ����������III�е�ƽ�ⳣ��
C. ƽ��ʱSO3�����ʵ�����a��1.8��b��1.8
D. ����ʼʱ������I�г���0.10 mol SO2(g)��0.20mol O2(g)��2.0 mol SO3(g)�����ʱv����v��