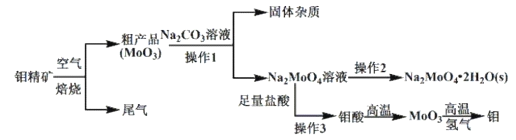
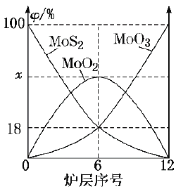
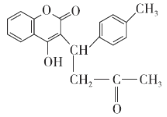
��Ŀ����
����Ŀ������˵�����ʾ����ȷ���ǣ� ��
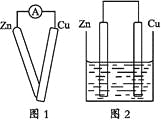
A.���������������������ֱ���ȫȼ�գ�ǰ�߷ų���������
B.C�����ʯ��s����C��ʯī��s�� ��H����1.19kJ��mol��1������ʯī�Ƚ��ʯ�ȶ�
C.��1mol H2SO4��Ũ������������NaOH��Һ��Ӧ���ų�����Ϊ57.3kJ
D.��101 kPa�£�2 g H2 ��ȫȼ������Һ̬ˮ�ų�285.8kJ���������Ȼ�ѧ����ʽΪH2(g)��![]() O2
O2![]() H2O(l) ��H����285.8kJ��mol��1
H2O(l) ��H����285.8kJ��mol��1
���𰸡�C
��������
A. ���������ߣ����������������������ֱ���ȫȼ�գ��������ų��������϶࣬A����ȷ��
B. ����Խ�ͣ�����Խ�ȶ�������ʯī�Ƚ��ʯ�ȶ���B����ȷ��
C. ϡ�������������Ʒ�Ӧ����1molˮʱ���ų���������57.3kJ��Ũ��������ˮ��C�����
D. ȼ����ָ����1mol��������ȫȼ�������ȶ�������ʱ�ų���������1mol������ȫȼ�գ��ų�285.8 kJ����, �Ȼ�ѧ����ʽΪH2(g)��![]() O2
O2![]() H2O(l) ��H����285.8kJ��mol��1��D����ȷ��
H2O(l) ��H����285.8kJ��mol��1��D����ȷ��
��ѡC��

��ϰ��ϵ�д�
 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д�
�����Ŀ