��Ŀ����
18��һ����̼������ʽCO������ɫ����ζ���壬�綾���ȿ������ᣮ��ˮ�е��ܽ�Ⱥ�С��һ����̼�Ǻ�̼���ʲ���ȫȼ�յIJ��Ҳ������Ϊȼ��ʹ�ã�ú��ˮ�ڸ����¿�������ˮú����һ����̼�������Ļ�������Щ�ִ������������������������Ʒһ����̼����Ϊ�������壮ȼ��ʱ������ɫ�Ļ��棬�ų��������ȣ����һ����̼������Ϊ����ȼ�ϣ�һ����̼��Ϊ��ԭ�������»����ʱ�ܽ�������������ﻹԭ�ɽ������ʣ���˳����ڽ�����ұ��������һ����̼������Ѫ�쵰������������Ѫ�쵰��������200-300������̼��Ѫ�쵰������Ѫ�쵰�Ľ����ٶ���3600������һ����̼Ũ���ڿ����дﵽ35ppm���ͻ����������������һ����̼�ж���
��ҵ�����֡���������Ҥ��������������¯�Ż�Ҥ�Źرղ��ϣ�ú���ܵ�©���������ų�β���������ݳ�������һ����̼������������ú¯����ȡů��ȼ�ղ���ȫ������һ����̼��
һ����̼�ж���֢״��ͷ�Ρ�ͷʹ�����ġ�Ż�¡���Ӧ�ٶۣ�����ʱ��������ԣ������ֻ�������Ϊһ����̼�ж�ʱ��Ӧ������ȡ������ʩ���������Ŵ�ͨ�磻ȷ��������ͨ����Ѹ�������и�ѹ������������ҽԺ��
��1��һ����̼����������Ϊ��ɫ����ζ���壬�ȿ������ᣬ��ˮ�е��ܽ�Ⱥ�С��
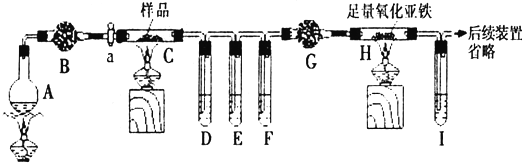
��2��Ϊ��Ԥ��ú���ж���������ú¯ȡů�ļ�ͥ���д�ʩ��ȷ����b��
a����ú¯��ͷ��һ����ˮ b���̵����Ӵ��ý����ܷ�
��3��CO�ж���ԭ����һ����̼������Ѫ�쵰������������Ѫ�쵰��������200-300����
��4����ҵ��ú��ˮ����ˮú���Ļ�ѧ����ʽ��C+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2��
��5��CO���ڹ�ҵ������д���˷�Ӧ�Ļ�ѧ����ʽFe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2��
���� ��1�����ʵĻ�ѧ������ָ�ڻ�ѧ�仯�б��ֳ��������ʣ����ʵ�����������ָ����Ҫͨ����ѧ�仯���ֳ��������ʣ�
��2��һ����̼������ˮ���ܷ��̵��ɷ�ֹһ����̼й©��
��3��������Ŀ��Ϣ������һ����̼������Ѫ�쵰������������Ѫ�쵰��������200-300������̼��Ѫ�쵰������Ѫ�쵰�Ľ����ٶ���3600������һ����̼Ũ���ڿ����дﵽ35ppm���ͻ����������������һ����̼�ж����
��4����ˮ����ͨ�����ȵ�ú����ƵýϽྻ��ˮú������Ҫ�ɷ���CO��H2����
��5��һ����̼���л�ԭ�ԣ���ԭ������������ͬʱ���ɶ�����̼��
��� �⣺��1�����ʵ���ɫ���ܶȡ������ԡ��ܽ��ԡ�״̬��Ӳ�ȡ�����ȷ�������ʲ���Ҫͨ����ѧ�仯���ֳ����������������ʣ�������Ϣ��֪��һ����̼����������Ϊ��ɫ����ζ���壬�ȿ������ᣬ��ˮ�е��ܽ�Ⱥ�С��
�ʴ�Ϊ����ɫ����ζ���壬�ȿ������ᣬ��ˮ�е��ܽ�Ⱥ�С��
��2��a��ú���ж���ָһ����̼�ж���һ����̼���������ڵ�Ѫ�쵰��ϣ�ʹ��ʧȥ����������ʹ����ȱ�����ж���һ����̼������ˮ����¯���Ϸ�һ����ˮ����Ԥ��ú���ж�����a����
b���̵����Ӵ��ý����ܷ⣬��ֹһ����̼й©����b��ȷ��
�ʴ�Ϊ��b��
��3������һ����̼������Ѫ�쵰������������Ѫ�쵰��������200-300������̼��Ѫ�쵰������Ѫ�쵰�Ľ����ٶ���3600������һ����̼Ũ���ڿ����дﵽ35ppm���ͻ����������������һ����̼�ж���
�ʴ�Ϊ��һ����̼������Ѫ�쵰������������Ѫ�쵰��������200-300����
��4��ú����Ҫ�ɷ�Ϊ̼��̼��ˮ������ӦC+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2���ʴ�Ϊ��C+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2��
��5��һ����̼���л�ԭ�ԣ���ԭ��������Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2���ʴ�Ϊ��Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2��
���� ���⿼����CO���й�֪ʶ������һ����̼���ж�ԭ������ԭ�ԡ��������ʵ�ʽ���ؼ���ע�������Ϣ����ȡ����Ŀ�ѶȲ���

| A�� | 56g N2��12g H2���ܱ������з�Ӧ��ת�Ƶ��ӵ���ĿΪ12.0��6.02��1023�� | |
| B�� | ��״���£�22.4L NO��11.2L O2��Ϻ�����ķ�������Ϊ6.02��1023 | |
| C�� | ��ҵ���õ�ⷨ���д�ͭ����ʱ��ÿת��1mol���ӣ��������ܽ��ͭԭ�ӱ�Ϊ0.5��6.02��1023 | |
| D�� | V L a mol•L-1 �Ȼ�����Һ�У���Fe3+����ĿΪ6.02��1023����Cl-����Ŀ����3��6.02��1023 |
| A�� | ʵ���������� | B�� | ������� | C�� | �������� | D�� | ������ |
| A�� | ���� | B�� | �� | C�� | �ط� | D�� | ���Ϸ� |
| A�� | ���á��������������ȼú����������β��������������߿������� | |
| B�� | ���������������ˮ��Ӧ���ɶ�Ӧ���ᣬ��CO2��SiO2��SO3 | |
| C�� | ʯ�ͻ����е��ѻ����ѽ���̶���ͨ����ѧ��Ӧ�������̬ϩ�� | |
| D�� | �Ҵ����������⡢�������ƵȾ�ͨ���������ôﵽɱ��������Ŀ�� |
| A�� | ��ͥ�õġ�84������Һ�����鲻��ͬʱ���ʹ�ã�����ᷢ���ж��¹� | |
| B�� | ���������ǹ��ұ����İ���������ʼ�������֮�ƣ���֪�ò����Ի�ѧҩƷ���У����Դ���������Һ���Խ������������ﵽ������Ŀ�� | |
| C�� | �Ȼ�����HgCl2����ϡ��Һ������������е��������Ϊ����ʹ�����ʱ��ԣ�ɱ������ | |
| D�� | ú����Ҫ�ɷ�Ϊ����̼���������ױ��ȣ������ͨ��ú�ĸ������Ƿ��� |
| A�� |  ����װ�������� | B�� |  �ռ����� | ||
| C�� | 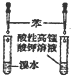 ��֤���в�����̼̼˫�� | D�� |  ʵ������ȡ���ռ����� |
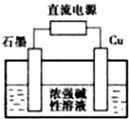 �������ʼ��仯�����ڹ�ũҵ������Ӧ�ù㷺��
�������ʼ��仯�����ڹ�ũҵ������Ӧ�ù㷺��