��Ŀ����
����Ŀ�����ຬ�����ʻ���ɻ�����Ⱦ�����Ṥҵ��β��(��NO��NO2)����ɴ�����Ⱦ��ͨ����ѡ���ԷǴ���ԭ�����Һ���շ�[ʯ�������գ����ܾ���β�������ܻ��Ӧ�ù㷺��Ca(NO2)2]������ˮ�й����İ���(NH3��NH4+)�ᵼ��ˮ�帻Ӫ��������ͨ����ǿ�����������ա�
(1)�����е�����������ա�
��ѡ���ԷǴ���ԭ������������Ҫ��Ӧ�У�
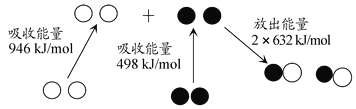
4NH3(g)+4NO(g)+O2(g)![]() 4N2(g)+6H2O(g)����H=-1646 kJ��mol-1
4N2(g)+6H2O(g)����H=-1646 kJ��mol-1
2NO(g)+(NH2)2CO(s)+![]() O2(g)
O2(g)![]() 2N2(g)+2H2O(g)+CO2(g) ��H=��780.02 kJ��mol-1
2N2(g)+2H2O(g)+CO2(g) ��H=��780.02 kJ��mol-1
��2NH3(g)+CO2(g)![]() CO(NH2)2(s)+H2O(g)����H=__________��
CO(NH2)2(s)+H2O(g)����H=__________��
�ڼ�Һ���շ����ù����в�����Һ�����Ӵ�����(β�������������룬ʯ�����������������)���õ���������ѭ��ʹ�ã�����������Ҫ�ɷ���________(�ѧʽ)�� ���������NO��NO2���ʵ���֮�Ƚӽ�__________����С�������ֵ����ᵼ��_______________��
(2)��ˮ�й����İ���(NH3��NH4+)������ij����С����NaClO����������������ˮ��
��֪��A.HClO�������Ա�NaClOǿ��
B.NH3��NH4+���ױ�������
C.���ұ�Ҫ�������İ�����ˮpHҪ������6��9��
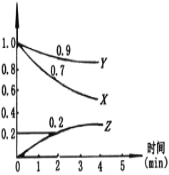
��ˮpH����ȥ���ʵ�Ӱ������ͼ��ʾ��
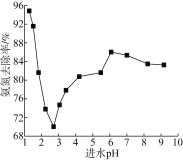
�ٽ�ˮpHΪ2.75��6.00��Χ�ڣ�����ȥ������pH���߶�������ԭ����___________��
��д������������ClO-����NH4+�����ӷ���ʽ____________________��
����֪a.O2�������Ա�NaClO���� b.O2�����������ʱ�NaClO����
��������������ʱ�������ӵ�λʱ����ͨ���������������ȥ����_______________(���������������������С����������������)��
���𰸡���42.98 kJ/mol Ca(OH)2 1��1 Ca(NO2)2������Ca(NO3)2�ĺ���ƫ�� ����pH���ߣ�������ˮ��NH3�������������ױ����� 3ClO-+2NH4+=N2��+3Cl-+3H2O+2H+(��дΪ3HClO+2NH4+=N2��+3Cl-+3H2O+5H+) ��������
��������
(1)�ٸ��ݸ�˹���ɣ�����֪�Ȼ�ѧ����ʽ���ӣ��ɵ�2NH3(g)+CO2(g)![]() CO(NH2)2(s)+H2O(g)�ķ�Ӧ�ȣ�
CO(NH2)2(s)+H2O(g)�ķ�Ӧ�ȣ�
�ڸ������Ṥҵ��β��(��NO��NO2)��Ca(OH)2��Ӧ����Ca(NO2)2��ʯ�����к�����Ca(OH)2��������Ca(OH)2�ᵼ�����ʲ��������NO2��Ca(OH)2��Ӧ���������ʳɷַ������������NO��NO2���ʵ���֮�ȼ����ã�
(2)�ٸ��ݷ�ˮ����ҺpH��С�����к��е�NH3��NH4+�Ķ��ټ�NH3��NH4+���ױ�����������
������������ClO-����NH4+ΪN2��ClO-����ԭ����Cl-��
�۸�����֪����a. O2�������Ա�NaClO����
b. O2�����������ʱ�NaClO�����з����жϡ�
(1)����֪��Ӧ��(i)4NH3(g)+4NO(g)+O2(g)![]() 4N2(g)+6H2O(g) ��H=-1646 kJ/mol��
4N2(g)+6H2O(g) ��H=-1646 kJ/mol��
(ii)2NO(g)+(NH2)2CO(s)+![]() O2(g)
O2(g)![]() 2N2(g)+2H2O(g)+CO2(g) ��H=-780.02 kJ/mol��
2N2(g)+2H2O(g)+CO2(g) ��H=-780.02 kJ/mol��
���ڷ�Ӧ�����е������仯ֻ�����ʵ�ʼ̬����̬�йأ��뷴Ӧ�����أ����ݸ�˹���ɣ���(i)��![]() -(ii)�������ɵ�2NH3(g)+CO2(g)
-(ii)�������ɵ�2NH3(g)+CO2(g)![]() CO(NH2)2(s)+H2O(g)�ķ�Ӧ����H= -42.98 kJ/mol��
CO(NH2)2(s)+H2O(g)�ķ�Ӧ����H= -42.98 kJ/mol��
��NO��NO2��Ca(OH)2�ᷢ�����з�Ӧ��NO+NO2+Ca(OH)2=Ca(NO2)2+H2O������Ca(NO2)2������ˮ��Ca(OH)2����ˮ����ʯ������Ca(OH)2��Ҫ�Թ�����ʽ���ڣ����²����������ֽӴ���ʹ���õ��������к���Ca(OH)2�����ݷ���ʽNO+NO2+Ca(OH)2=Ca(NO2)2+H2O��֪��Ҫ��ȡCa(NO2)2��Ӧ�ÿ���NO��NO2�����ʵ����ı���1��1����С�������ֵ����NO2����������ᷢ����Ӧ��4NO2+2Ca(OH)2=Ca(NO3)2+Ca(NO2)2+2H2O��ʹCa(NO2)2������Ca(NO3)2�ĺ���ƫ�ߣ������������ֵ��NO������ȫ�����գ���Ȼ�ᵼ�´�����Ⱦ��
(2)�ٷ�ˮ����ҺpHԽ�����к��е�NH3������Խ�ߣ�����NH3��NH4+���ױ���������˰������ױ�������
��������������ClO-��NH4+����������ԭ��Ӧ����N2��Cl-�����ݵ����غ㡢����غ㡢ԭ���غ㣬�ɵø÷�Ӧ�����ӷ���ʽΪ��3ClO-+2NH4+=N2��+3Cl-+3H2O+2H+(��дΪ3HClO+2NH4+=N2��+3Cl-+3H2O+5H+)��
������a.O2�������Ա�NaClO����b.O2�����������ʱ�NaClO���������������������ʱ�������ӵ�λʱ����ͨ���������������ȥ���ʼ������䡣

 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�