题目内容
【题目】工业废气中的二氧化硫和氮氧化物是大气主要污染物,脱硫脱氮是环境治理的热点问题。回答下列问题:
(1)已知氮及其化合物发生如下反应:
N2(g)+O2(g)![]() 2NO(g) ΔH=+180kJ·mol-1
2NO(g) ΔH=+180kJ·mol-1
N2(g)+2O2(g)![]() 2NO2(g) ΔH=+68kJ·mol-1
2NO2(g) ΔH=+68kJ·mol-1
则2NO(g)+O2(g)![]() 2NO2(g)ΔH___kJ·mol-1。
2NO2(g)ΔH___kJ·mol-1。
(2)利用KMnO4脱除二氧化硫的离子方程式为:___
①□MnO![]() +□SO2+□H2O
+□SO2+□H2O![]() □MnO
□MnO![]() +□ +□ 。(在“□”里填入系数,在“__”上填入微粒符号)
+□ +□ 。(在“□”里填入系数,在“__”上填入微粒符号)
②在上述反应中加入CaCO3可以提高SO2去除率,原因是___。
(3)CaSO3与Na2SO4混合浆液可用于脱除NO2,反应过程为:
I.CaSO3(s)+SO![]() (aq)
(aq)![]() CaSO4(s)+SO
CaSO4(s)+SO![]() (aq)
(aq)
II.SO![]() (aq)+2NO2(g)+H2O(l)
(aq)+2NO2(g)+H2O(l)![]() SO
SO![]() (aq)+2NO
(aq)+2NO![]() (aq)+2H+(aq)
(aq)+2H+(aq)
浆液中CaSO3质量一定时,Na2SO4的质量与NO2的去除率变化趋势如图所示。

a点后NO2去除率降低的原因是___。
(4)检测烟道气中NOx含量的步骤如下:
I.将VL气样通入适量酸化的H2O2溶液中,使NOx完全被氧化为NO![]() ;
;
II.加水稀释至100.00mL,量取20.00mL该溶液,与V1mLc1mol·L-1FeSO4标准溶液(过量)充分混合;
III.用c2mol·L-1KMnO4标准溶液滴定剩余的Fe2+,终点时消耗V2mL。
①NO被H2O2氧化为NO![]() 的离子方程式为___。
的离子方程式为___。
②滴定过程中主要使用的玻璃仪器有___和锥形瓶等。
③滴定过程中发生下列反应:
3Fe2++NO+4H+=NO↑+3Fe3++2H2O
MnO![]() +5Fe2++8H+=Mn2++5Fe3++4H2O
+5Fe2++8H+=Mn2++5Fe3++4H2O
烟气气样中NOx折合成NO2的含量为__mg·m-3。
【答案】-112 2MnO4-+1SO2+2H2O![]() 2MnO42-+ SO42-+4H+ CaCO3与H+作用,c(H+)下降,同时生成的Ca2+与SO42-结合生成CaSO4使得c(SO42-)下降平衡正向移动,从而提高SO2的去除率 a点后c(SO42-)过高时,以反应II平衡的逆向移动为主,NO2去除率降低 2NO+3H2O2=2NO3-+2H++2H2O 酸式滴定管
2MnO42-+ SO42-+4H+ CaCO3与H+作用,c(H+)下降,同时生成的Ca2+与SO42-结合生成CaSO4使得c(SO42-)下降平衡正向移动,从而提高SO2的去除率 a点后c(SO42-)过高时,以反应II平衡的逆向移动为主,NO2去除率降低 2NO+3H2O2=2NO3-+2H++2H2O 酸式滴定管 ![]()
【解析】
(1)由盖斯定律计算可得;
(2)①二氧化硫与高锰酸钾溶液发生氧化还原反应生成锰酸锰、硫酸钾、硫酸和水;
②向反应中加入CaCO3,CaCO3与反应生成的H+和SO42-反应,使H+和SO42-的浓度减小,使平衡向正反应方向移动;
(3)由反应II可知,若硫酸根浓度过大,平衡向逆反应方向移动,二氧化氮的浓度增大;
(4)①一氧化氮与过氧化氢发生氧化还原反应生成硝酸和水;
②KMnO4标准溶液具有强氧化性,会腐蚀橡胶管;
③由得失电子数目守恒和氮原子个数守恒计算可得。
(1)将已知反应依次编号为①②,由盖斯定律可知,②—①得一氧化氮与氧气反应的热化学方程式2NO(g)+O2(g)![]() 2NO2(g),则ΔH=(+68kJ·mol-1)—(+180kJ·mol-1)=-112 kJ·mol-1,故答案为:-112;
2NO2(g),则ΔH=(+68kJ·mol-1)—(+180kJ·mol-1)=-112 kJ·mol-1,故答案为:-112;
(2)①二氧化硫具有还原性,高锰酸钾溶液具有强氧化性,二氧化硫与高锰酸钾溶液发生氧化还原反应生成锰酸锰、硫酸钾、硫酸和水,则配平的离子方程式为2MnO4-+1SO2+2H2O![]() 2MnO42-+SO42-+4H+,故答案为:2MnO4-+1SO2+2H2O
2MnO42-+SO42-+4H+,故答案为:2MnO4-+1SO2+2H2O![]() 2MnO42-+SO42-+4H+;
2MnO42-+SO42-+4H+;
②向反应中加入CaCO3,CaCO3与反应生成的H+和SO42-反应,使H+和SO42-的浓度减小,使平衡向正反应方向移动,从而可以提高SO2去除率,故答案为:CaCO3与H+作用,c(H+)下降,同时生成的Ca2+与SO42—结合生成CaSO4使得c(SO42-)下降平衡正向移动,从而提高SO2的去除率;
(3)由反应II可知,若硫酸根浓度过大,平衡向逆反应方向移动,二氧化氮的浓度增大,去除率下降,故答案为:a点后c(SO42-)过高时,以反应II平衡的逆向移动为主,NO2去除率降低;
(4)①一氧化氮具有还原性,过氧化氢具有氧化性,一氧化氮与过氧化氢发生氧化还原反应生成硝酸和水,反应的离子方程式为2NO+3H2O2=2NO3-+2H++2H2O,故答案为:2NO+3H2O2=2NO3-+2H++2H2O;
②KMnO4标准溶液具有强氧化性,会腐蚀橡胶管,所以滴定过程中应选用酸式滴定管,故答案为:酸式滴定管;
③由得失电子数目守恒可得:n(Fe2+)= 3n(NO3-)+5n(MnO4-),n(NO3-)=![]() ,由氮原子个数守恒可知n(NO2)= n(NO3-)=
,由氮原子个数守恒可知n(NO2)= n(NO3-)=![]() mol,则烟气气样中NOx折合成NO2的含量为
mol,则烟气气样中NOx折合成NO2的含量为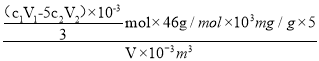 =
=![]() mg·m-3,故答案为:
mg·m-3,故答案为:![]() 。
。

 计算高手系列答案
计算高手系列答案【题目】 CO2的资源化利用一直是化学家们关注的重要课题,中科院大连化学物理研究所设计了一种新型多功能复合催化剂,成功地实现了CO2直接加氢制取高辛烷值汽油:![]() (反应①),该研究成果被评价为“CO2催化转化领域的突破性进展”。
(反应①),该研究成果被评价为“CO2催化转化领域的突破性进展”。
(1)已知氢气的燃烧热为![]() ,若要利用
,若要利用![]() 的燃烧热求a的值,则还需要知道一个反应的
的燃烧热求a的值,则还需要知道一个反应的![]() ,该反应是________________________________。反应①在一定条件下具有自发性,则a_______________0(填“>”或“<”)。
,该反应是________________________________。反应①在一定条件下具有自发性,则a_______________0(填“>”或“<”)。
(2)向某密闭容器中按一定投料比充入![]() 、
、![]() ,控制条件使其发生反应:
,控制条件使其发生反应:![]() 。测得
。测得![]() 的平衡转化率与温度、压强之间的关系如图1所示:
的平衡转化率与温度、压强之间的关系如图1所示:
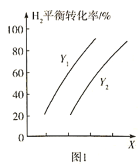
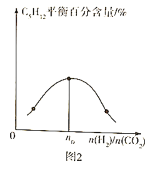
则X表示______________,![]() ___________
___________![]() (填“>”或“<”)。欲提高
(填“>”或“<”)。欲提高![]() 的平衡转化率并提高单位时间内
的平衡转化率并提高单位时间内![]() 的产量,可采取的措施是______________________(填两种)。
的产量,可采取的措施是______________________(填两种)。
(3)控制一定温度、催化剂,按不同投料比![]() 将反应物通入到某密闭容器中,测得平衡时
将反应物通入到某密闭容器中,测得平衡时![]() 的百分含量与投料比之间的关系如图2所示,则
的百分含量与投料比之间的关系如图2所示,则![]() ____________。
____________。
(4)在钌-铑双金属催化剂的作用下,CH3OH、CO2、H2可高效地转化为乙酸,反应方程式为![]() 。一定温度下,向某刚性容器中通入等物质的量的三种原料气,测得体系中的总压强与时间的关系如下表所示:
。一定温度下,向某刚性容器中通入等物质的量的三种原料气,测得体系中的总压强与时间的关系如下表所示:
t/min | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
p/kPa | 3 | 2.7 | 2.5 | 2.35 | 2.26 | 2.2 | 2.2 |
则反应开始到达到平衡的过程中,![]() ______________
______________![]() ______________
______________![]() 。
。
(5)碳捕捉技术的发展也有利于CO2在资源应用方面得到充分利用。常温下,若某次用NaOH溶液捕捉空气中的CO2所得溶液的pH=10,并测得溶液中![]() ,则
,则![]() _____________
_____________![]() 。
。
【题目】碘在科研与生活中有重要应用。某兴趣小组用0.2 %淀粉溶液、0.50 mol·L-1KI、0.20 mol·L-1K2S2O8、0.10 mol·L-1Na2S2O3等试剂,探究反应条件对化学反应速率的影响。已知:S2O82-+2I-=2SO42-+I2(慢) I2+2 S2O32-=2I-+S4O62-(快)
(1)为探究反应物浓度对化学反应速率的影响,设计的实验方案如下表:
实验序号 | 体积V/mL | ||||
K2S2O8溶液 | 水 | KI溶液 | Na2S2O3溶液 | 淀粉溶液 | |
10.0 | 0.0 | 4.0 | 4.0 | 2.0 | |
9.0 | 1.0 | 4.0 | 4.0 | 2.0 | |
8.0 | V8 | 4.0 | 4.0 | 2.0 | |
表示V8=________mL,理由是__________________。
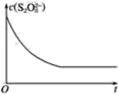
(2)已知某条件下,浓度c(S2O82-)-反应时间t的变化曲线如图所示,若保持其他条件不变,请在坐标图中,分别画出降低反应温度和加入催化剂时c(S2O82-)-t的变化曲线示意图(进行相应的标注)。_________
【题目】在100℃时,将0.200 mol的四氧化二氮气体充入2 L真空的密闭容器中,每隔一定的时间对该容器内的物质进行分析,得到如下表格:
| 0 | 20 | 40 | 60 | 80 | 100 |
c(N2O4) | 0.100 | c1 | 0.050 | c3 | a | b |
c(NO2) | 0.000 | 0.060 | c2 | 0.120 | 0.120 | 0.120 |
试填空:
(1)该反应的化学方程式为N2O4![]() 2NO2 ,达到平衡时,四氧化二氮的转化率为__________%,表中c2________c3、a______b(填“>”、“<”或“=”)。
2NO2 ,达到平衡时,四氧化二氮的转化率为__________%,表中c2________c3、a______b(填“>”、“<”或“=”)。
(2) 20 s时四氧化二氮的浓度c1=________mol/L,在0 s~20 s时间段内,四氧化二氮的平均反应速率为________mol/(L·s)。
(3)若在相同情况下最初向该容器充入的是二氧化氮气体,要达到上述同样的平衡状态,二氧化氮的起始浓度是________mol/L。
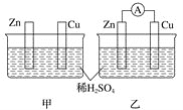
【题目】已知反应![]() 是可逆反应。设计如图装置(
是可逆反应。设计如图装置(![]() 均为石墨电极),分别进行下述操作:
均为石墨电极),分别进行下述操作:
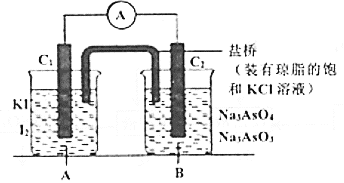
①向B烧杯中逐滴加入浓盐酸。②向B烧杯中逐滴加入![]() 溶液。结果发现电流表指针均发生偏转。
溶液。结果发现电流表指针均发生偏转。
(1)①过程中![]() 棒上发生的反应为_________;
棒上发生的反应为_________;
②过程中![]() 棒上发生的反应为___________。
棒上发生的反应为___________。
(2)操作②过程中,盐桥中的![]() 移向_________烧杯溶液(填“A”或“B”)。
移向_________烧杯溶液(填“A”或“B”)。
资料:![]() 。向
。向![]() 一定浓度的
一定浓度的![]() 溶液中加入
溶液中加入![]() 溶液,达平衡后,相关微粒浓度如下:
溶液,达平衡后,相关微粒浓度如下:
微粒 |
|
|
|
浓度 |
|
| a |
(3)a=________________。该平衡体系中除了含有![]() 和
和![]() 外,判断溶液是否一定还含有其他含碘微粒______________(填“是”或“否”)。
外,判断溶液是否一定还含有其他含碘微粒______________(填“是”或“否”)。
(4)已知:甲醇与水蒸气重整制氢可直接用于燃料电池。
反应:![]()
反应:![]()
则![]() ______________
______________![]()
(5)已知:25℃时,![]() 。医学上进行消化系统的X射线透视时,常使用
。医学上进行消化系统的X射线透视时,常使用![]() 作内服造影剂。胃酸酸性很强(pH约为1),但服用大量
作内服造影剂。胃酸酸性很强(pH约为1),但服用大量![]() 仍然是安全的,
仍然是安全的,![]() 不溶于酸的原因是(用溶解平衡原理解释):_________。误服少量
不溶于酸的原因是(用溶解平衡原理解释):_________。误服少量![]() ,应尽快用大量的一定浓度的
,应尽快用大量的一定浓度的![]() 溶液给患者洗胃,忽略洗胃过程中
溶液给患者洗胃,忽略洗胃过程中![]() 溶液浓度的变化,要使残留在胃液中的
溶液浓度的变化,要使残留在胃液中的![]() 浓度为
浓度为![]() ,应服用的
,应服用的![]() 溶液的最低浓度为_______
溶液的最低浓度为_______![]() 。
。