��Ŀ����
��Ȼ��������Ҫ����Դ��Ҳ����Ҫ�Ļ���ԭ�ϣ�����Ҫ�ɷ��Ǽ��顣
��1��������һ�������¿����ɣ�
A��̼�����ӣ�CH3-������������ B��̼�����ӣ�CH3-��
C��������CH3�� D��̼ϩ��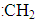 ��
��
���������У�����Ϊ120�����________��������ţ�
����̼�����ӣ�CH3-����Ϊ�ȵ������һ�ַ��ӵĽṹʽΪ_________________________________________________________��
��2�������ش����Ŀ�ȼ���������ˮ��������ڿ�ȼ����˵������ȷ����________��
A�����������ˮ���Ӿ��Ǽ��Է���
B����ȼ���м��������ˮ���Ӽ����������
C����ȼ������ԭ�Ӿ���
D�����������ˮ�����еĦҼ�����s��sp3�������ص����ɵ�
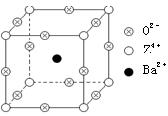
��3���ڸ����£�����ɻ�ԭCuO�õ�Cu2O��
��Cu���ĺ�������Ų�ʽΪ____________��
��Cu2O����ľ����ṹ��ͼ��ʾ�����С��������������ӷ���Ϊ________��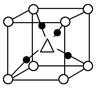
��4��һ�������£�������ˮ����������H2��CO���������ɵ������ЦҼ�������м�����֮��Ϊ________��
��1����A����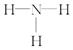 ����2��D����3����[Ar]3d10
����2��D����3����[Ar]3d10
��O2������4��2��1
����

NaF��NaI����MgO��Ϊ���Ӿ��壬�й��������±���
| �� �� | �� NaF | �� NaI | �� MgO |
| ���ӵ���� | 1 | 1 | 2 |
| ����(10-10m) | 2.31 | 3.18 | 2.10 |
���жϣ������ֻ������۵��ɸߵ��͵�˳����
A���ۣ��٣��� B���ۣ��ڣ��� C���٣��ڣ��� D���ڣ��٣���
���и����У����ɡ����ۼ������ϵ����ȷ����
| ѡ�� | ���� | ���� |
| A | ���ܣ�N��N��Cl��Cl | ���ʷе㣺N2��Cl2 |
| B | �����пɵ����H+������H2SO4��CH3COOH | ���ԣ�H2SO4��CH3COOH |
| C | Ԫ�صķǽ����ԣ�N��P | ���ԣ�HNO3��H3PO4 |
| D | �����ԣ�Fe3+��Cu2+ | ��ԭ�ԣ�Fe2+��Cu |
��֪��
(1)����ʧˮ���Ȼ�ѧ����ʽΪCuSO4��5H2O(s)===CuSO4(s)��5H2O(l) ��H����Q1 kJ/mol
(2)�����£���ˮ����ͭ����ˮ���Ȼ�ѧ����ʽΪCuSO4(s)===Cu2��(aq)��SO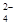 (aq) ��H��
(aq) ��H��
��Q2 kJ/mol
(3)����(CuSO4��5H2O)����ˮʱ��Һ�¶Ƚ��͡���Q1��Q2�Ĺ�ϵ��(Q1��Q2Ϊ����) �� ��
| A��Q1>Q2 | B��Q1��Q2 | C��Q1<Q2 | D����ȷ�� |
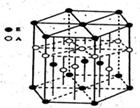
���о��尴A1�ͷ�ʽ���н��ܶѻ����ǣ� ����
| A���ɱ���NaCl������ͭ |
| B��ZnS������þ�������� |
| C��ˮ�������ʯ������� |
| D��ZnS��NaCl������þ |
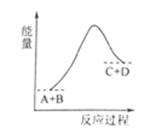
 C+D�������仯��ͼ��ʾ������˵����ȷ����
C+D�������仯��ͼ��ʾ������˵����ȷ����