��Ŀ����
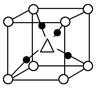
��.��֪���н������壺Na��Po��K��Fe��Cu��Mg��Zn��Au����ѻ���ʽΪ��
��1���������ѻ�����____________________________________________��
��2�����͵���______________________________________________________��
��3��þ�͵���______________________________________________________��
��4��ͭ�͵���_____________________________________________________��
��.A��B��C��D���Ƕ�����Ԫ�أ�ԭ�Ӱ뾶D>C>A>B����֪��A��B����ͬһ���ڣ�A��C����ͬһ���壻Cԭ�Ӻ��ڵ�����������A��Bԭ�Ӻ��ڵ�������֮�ͣ�Cԭ��������������Dԭ��������������4����
�Իش�
��1��������Ԫ�طֱ��ǣ�A______��B______��C______��D______����Ԫ�����ƣ���
��2��������Ԫ�ص��ʵ��۵��ɸߵ��͵�˳����________����Ԫ�����ƣ���
��3��C�Ĺ�̬��������________���壬D�Ĺ�̬������________���塣
��4��д��A��B��D��ɵĻ�������B��C��ɵĻ��������Ӧ�Ļ�ѧ����ʽ_______________________________________________________________��
��.��1��Po
��2��Na��K��Fe
��3��Mg��Zn
��4��Au��Cu
��.��1��̼�������衡��
��2��̼>��>��>��
��3��ԭ�ӡ�����
��4��Na2CO3��SiO2 Na2SiO3��CO2��
Na2SiO3��CO2��
����

 �Ķ��쳵ϵ�д�
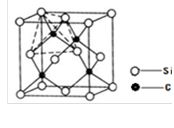
�Ķ��쳵ϵ�д���̼ԭ�ӹ��ɵ�����̼�ܿ�����Ϊ���������������������䵥�㲿�ֽṹʾ��ͼ���£�����˵����ȷ����
| A������̼����һ���������� |
| B������̼����̼ԭ�Ӽ��Թ��ۼ���� |
| C������̼����ʯī��Ϊͬλ�� |
| D��0.12g����̼���к���6.02��l022��̼ԭ�� |
���ʵ��۷е�ߵ����ü��ܴ�С���͵���
| A��Na �� K | B��O2�� N2 | C��H2O �� H2S | D��SiO2 �� CO2 |
���ڻ�ѧ��Ӧ�е������仯������˵���в���ȷ����( )
| A��ȼ�շ�Ӧ���Ƿ��ȷ�Ӧ |
B�����ڿ��淴Ӧ��aA(g)+bB(g) bC(g)+dD(g)���������Ӧ���ȣ��淴Ӧһ������ bC(g)+dD(g)���������Ӧ���ȣ��淴Ӧһ������ |
| C������ȼ������ˮ��һ�����ȵĻ�ѧ��Ӧ��˵��1 mol H2����������1 mol H2O������ |
| D��ֻ�з��ȵ�������ԭ��Ӧ�ſ������Ϊԭ��� |
SF6��һ�������ľ�Ե���壬���ӽṹ��ֻ����S-F������֪�� 1molS(s)ת��Ϊ��̬��ԭ����������280kJ,����1molF-F ��S-F�������յ������ֱ�Ϊ160kJ��330kJ����S(s)��3F2(g)=SF6(g)�ķ�Ӧ�ȡ�HΪ
| A��-1780kJ/mol | B��+1220 kJ/mol | C��-1220 kJ/mol | D��+1780 kJ/mol |
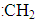 ��
��