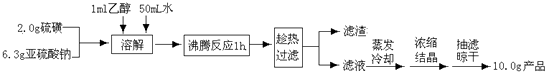
��Ŀ����
8��ij���᳧��������Ϊԭ����������Ĺ������£�| �������� | ��ѧ��Ӧ | ��Ӧ��� |
| ����������� | 4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2 | 4%����Ԫ����ʧ������¯�� |
| ������ | 2SO2+O2$?_{��}^{����}$2SO3 | SO2ת����Ϊ90% |
| SO3������ | SO3+H2O=H2SO4 | SO3������Ϊ100% |
��2������Cu��CuO�Ļ����20.8g���뵽50mL 18.4mol•L-1��ŨH2SO4�У����ȳ�ַ�Ӧ������������ȫ�ܽ⣬��ȴ���Һϡ����1000mL�������Һ��c��Cu2+��=0.3mol•L-1���Լ��㣺
�ٷ�Ӧ�ų��������ڱ�״���µ������������������ܽ⣩��4.48L��
��ϡ�ͺ����Һ�к��е�c��H+����0.84mol/L��
���� ��1������FeS2��2S��2H2SO4���㣻
��2���ٷֱ����Cu��CuO�����ʵ�������������Ϊ20.8g�ͷ�Ӧ��c��Cu2+��=0.3mol/L�з�������Cu��CuO�����ʵ�����Cu��Ũ���ᷴӦ�ų����壬���ݷ�Ӧ���㼴�ɣ�
���������������ӵ�������ȥ��Cu��CuO���ĵ��ģ����������ϡ�ͺ�c��H+����
��� �⣺��1����Sԭ���غ��֪��FeS2��2S��2H2SO4����
FeS2��2S��2H2SO4��
120 2��98
100t��40% x��98%
$\frac{120}{100t��40%}=\frac{2��98}{x��98%}$�����x=108t��
�ʴ�Ϊ��108��
��2����������Cu�����ʵ���Ϊx��CuO�����ʵ���Ϊy��
���ݻ����Ϊ20.8g���У�64x+80y=20.8����
���ݻ��Һϡ����1000mL��c��Cu2+��=0.3mol/L�����У�x+y=0.3����
��٢ڵã�x=0.2mol��y=0.1mol��
��Cu��Ũ���ᷴӦ�ų�SO2���壬��������������ʵ���Ϊamol�����ݷ�Ӧ��
Cu+2H2SO4 ��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O
1 1
0.2mol amol
�ɵã�$\frac{1}{0.2}=\frac{1}{a}$��ã�a=0.2mol��
�����DZ���£������V=n•Vm=4.48L��
�ʴ�Ϊ��4.48L��
����֪n��H2SO4����=0.05L��18.4mol/L=0.92mol
n��H2SO4����Ӧ=2x+y=0.5mol
n��H2SO4����=0.92mol-0.5mol=0.42mol
�� c��H +��=0.42mol��2��1L=0.84mol/L
�ʴ�Ϊ��0.84mol/L��
���� ���⿼�������ʵ���Ũ�ȵ���ؼ��㣬���չ�ʽ��ʹ���ǽ���Ĺؼ����ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��ϩ�����Ӿ۷�Ӧ�Ƶþ���ϩ�߷��Ӳ��� | |
| B�� | �ü����������Ʊ�һ�ȼ��� | |
| C�� | �ñ���Ũ���ᡢŨ����Ϊԭ����ȡ������ | |
| D�� | ��ʯ��ʯ��ϡ���ᷴӦ�Ƶö�����̼ |
| A�� | ��Ӧ����Һ������Ũ�ȴ�С��ϵΪ��c��NO3-����c��Ag+����c��Cl-����c��I-�� | |
| B�� | ��Һ���Ȳ�������AgI���� | |
| C�� | AgCl��KSP����ֵΪ1.69��10-10 | |
| D�� | ����AgCl����Һ�еμ�KI��Һ����ɫ������ת��ɻ�ɫ���� |
��1�����������ʵ����Ʊ������в�Ҫ���ո�
| ��� | ʵ��Ŀ�� | ̼��/g | ����/g | ����/% |
| �� | Ϊ����ʵ�������� | 0.5 | 2.0 | 90.0 |
| �� | 0.5 | 2.0 | 36.0 | |
| �� | ̼��������Ӱ�� | 0.2 | 90.0 |

��3����С���ͼ2����0-t1ʱѹǿ����ԭ����������¼��裬������ɼ��������
����һ���������ⸯʴ���������壻
���������Ӧ����ʹ��ƿ���¶����ߣ�
��4��Ϊ��֤����һ��ijͬѧ����˼����ռ����������Ƿ���H2�ķ��������������һ��ʵ�鷽����֤����һ��
| A�� | 1 L 0.1 mol•L-1 CH3COOH��Һ�к��е�CH3COO-Ϊ0.1NA | |
| B�� | �����£�1 L 0.1 mol•L-1 NH4NO3��Һ�к��еĵ�ԭ����Ϊ0.2NA | |
| C�� | �ڷ�ӦKIO3+6HI�TKI+3I2+3H2O�У�ÿ����3mol I2��ͬʱת�Ƶĵ�����Ϊ6NA | |
| D�� | ���³�ѹ�£�22.4 L��ϩ�к��еĹ��õ��ӶԵ���ĿΪ6NA |
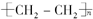 ?+3nO2$\stackrel{��ȼ}{��}$2nCO2+2nH2O��
?+3nO2$\stackrel{��ȼ}{��}$2nCO2+2nH2O��