��Ŀ����
17����Na2CO3•10H2O���壬����0.2mol/L��Na2CO3��Һ480mL����1��ʵ�����õ��IJ�����������Ͳ�����������ձ�����ȱ��500mL����ƿ����ͷ�ιܣ�
��2��Ӧ��������ƽ��ȡNa2CO3•10H2O�ľ��������Ϊ28.6g��
��3��������Һʱ�����¼���������
���ܽ⡡ ��ҡ�ȡ� ��ϴ�ӡ� ����ȴ�� �ݳ����� ��ת����Һ �߶���
��ȷ�IJ���˳���Ǣݢ٢ܢޢۢޢߢڣ�����ţ���
��4���������в�����������Һ��Ũ�ȸ���ʲôӰ�죬�����գ�
��̼����ʧȥ�˲��ֽᾧˮ�� ���á���������ij���������������
��̼���ƾ��岻�������л����Ȼ��� �ܳ���̼���ƾ���ʱ������������
������ƿδ�������ʹ��
��������������ҺŨ��ƫ�ߵ��Т٢ܣ���Ӱ����Тݣ�������ţ�
���� ��1���������Ʋ����Ǽ��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��2������n=cv��������Na2CO3�����ʵ���������Na2CO3•10H2O�����ʵ�������Na2CO3�����ʵ���������m=nM����Na2CO3•10H2O��������
��3���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���Բ���˳���������
��4������c=$\frac{n}{V}$�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��� �⣺��1������ƿ�Ĺ��û��480mL��ֻ����500mL����ƿ������0.2mol/L��Na2CO3��Һ500mL�����������м��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���������������������ƽ���ձ�����������500mL����ƿ����ͷ�ιܣ��ʻ�ȱ��500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��500 mL����ƿ����ͷ�ιܣ�
��2��ʵ������Ҫ0.2mol/L��Na2CO3��Һ480mL����������ƿ�Ĺ��û��480mL��ֻ����500mL����ƿ����500mLNa2CO3��Һ��ҪNa2CO3�����ʵ���Ϊ��0.5L��0.2mol/L=0.1mol��Na2CO3•10H2O�����ʵ���Ϊ0.1mol��Na2CO3•10H2O������Ϊ��0.1mol��286g/mol=28.6g��
�ʴ�Ϊ��28.6��
��3���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪��ȷ�IJ���˳���Ǣݢ٢ܢޢۢޢߢڣ�
�ʴ�Ϊ���ݢ٢ܢޢۢޢߢڣ�
��4����̼����ʧȥ�˲��ֽᾧˮ�����³������ʵ����������������ʵ����ʵ���ƫ��������Һ��Ũ��ƫ�ߣ���
���á���������ij��������������嵼�³������ʵ�����ƫС���������ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
��̼���ƾ��岻�������л����Ȼ��ƣ�����̼���Ƶ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
�ܳ���̼���ƾ���ʱ�����������⣬���³������ʵ����������������ʵ����ʵ���ƫ��������Һ��Ũ��ƫ�ߣ���
������ƿδ�������ʹ�ò�Ӱ�����ʵ����ʵ�����Ҳ��Ӱ����Һ����������Զ����Ƶ���ҺŨ����Ӱ�죻
��ѡ���٢ܣ��ݣ�
���� ���⿼��һ�����ʵ���Ũ����Һ�����ƣ��״����Ǽ������ʵ��������ܶ�ͬѧ����Һ�������Ϊ��480mL�����³�������Ŀ�Ѷ��еȣ�

| A�� | A��B���γ�AB32-��A2B42-������ | |
| B�� | B���⻯��ķе����C���⻯��ķе� | |
| C�� | ����C�ľ����к��й��ۼ��ͷ��»��� | |
| D�� | ��A��D�γɵ�һ�����ʿ�������ȡ��ˮ�еⵥ�� |
| �������� | ��ѧ��Ӧ | ��Ӧ��� |
| ����������� | 4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2 | 4%����Ԫ����ʧ������¯�� |
| ������ | 2SO2+O2$?_{��}^{����}$2SO3 | SO2ת����Ϊ90% |
| SO3������ | SO3+H2O=H2SO4 | SO3������Ϊ100% |
��2������Cu��CuO�Ļ����20.8g���뵽50mL 18.4mol•L-1��ŨH2SO4�У����ȳ�ַ�Ӧ������������ȫ�ܽ⣬��ȴ���Һϡ����1000mL�������Һ��c��Cu2+��=0.3mol•L-1���Լ��㣺
�ٷ�Ӧ�ų��������ڱ�״���µ������������������ܽ⣩��4.48L��
��ϡ�ͺ����Һ�к��е�c��H+����0.84mol/L��
| A�� | SO2��SO3���Ԫ����ͬ����H2O��Ӧ����Ҳ��ͬ | |
| B�� | Ũ��������ֽ⣬����ʱ��ʵ���ҿ�����Ũ����ʻ�ɫ | |
| C�� | CO��NO��NO2�����γɹ⻯ѧ�������Ǵ�����Ⱦ���壬�ڿ����ж����ȶ����� | |
| D�� | ������ˮ�����ԣ���������ˮ�еμ�������ɫʯ����Һ���������Һ�ʺ�ɫ |
| A�� | 1mol/L�� Fe2��SO4��3��Һ�к���2NA��Fe3+��������ˮ�⣩ | |
| B�� | 1mol�� Fe2��SO4��3��S2-��Ӧ��ת��2NA������ | |
| C�� | �ڸ���Һ�У�K+��NH4+��I-��SO42-���Դ������� | |
| D�� | ��Cu��Ӧ�����ӷ���ʽΪ��Fe3++Cu�TFe2++Cu2+ |
| A�� | ���ӻ�����һ���������ۼ� | |
| B�� | ���ۻ�����һ���������Ӽ� | |
| C�� | ��̬���ʵķ�����һ�����ڹ��ۼ� | |
| D�� | �ǽ���Ԫ�صĻ�����һ���������Ӽ� |
��һ���Ʊ�̼��������
��һ�������Ʊ�������������Һ������̼�������Һ��ϲ����������������壮
��1��д�����ӷ���ʽ��Fe2++2HCO3-=FeCO3��+CO2��+H2O��
������̽��̼�����������ȶ��ԣ���������ʡ�ԣ�
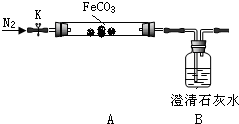
����������װҩƷ����K����ͨ��һ��ʱ�䵪����Ȼ���þƾ������A�����Ȳ����ܣ��۲�Bƿ��Һ����ǣ�������ֽ������ͨ�뵪������������ȴ��
��2����ͨ�뵪����Ŀ�����ž�װ���ڿ����������������ţ�Bƿ������˵���ֽ�����ж�����̼��
��3��ֹͣ����֮ǰ���Ƿ��A��B֮���ܣ��𣺷�������ͨ�뵪��������������ѹ�����С��
������̽��̼��������ԭ��
���������ϡ�������������һ�ֺ�ɫ��ĩ�������ȶ����ڿ����м��ȣ���Ѹ�ٱ���������������������̼�������ڿ���������������������
��4��̽��̼��������������Ӧ��Ĺ���ɷ֣�
��������롿����1 ����ɷ�����������
����2 ����ɷ���������������
����3��������������������
�����ʵ����֤����һ����ѡ�Լ���2.00mol/L��H2SO4��HCI��HNO3��KSCN��Һ��KMnO4��Һ��NaOH��Һ��H2O2��Һ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| �Լ� | FeCl3��Һ | ����KMnO4��Һ | NaHCO3��Һ |
| ���� | ��Һ����ɫ | ��ɫ | �ų����� |
| A�� | 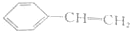 | B�� | 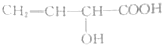 | ||
| C�� | 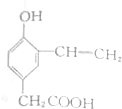 | D�� | 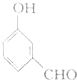 |

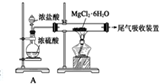 ������װ��A���������Ʊ������HCl����
������װ��A���������Ʊ������HCl����