��Ŀ����
����Ŀ����A��B��C��D��E���ֶ�����Ԫ�أ���֪���ڵ�A��B��C��D����Ԫ��ԭ�Ӻ����56�����ӣ������ڱ��е�λ����ͼ��ʾ��E�ĵ��ʿ����ᷴӦ��1molE���������������ã��ڱ�״�����ܲ���33.6L H2��E����������A�������Ӻ�����Ӳ�ṹ��ȫ��ͬ��
�ش��������⣺
��1��A��E�γɵĻ�����Ļ�ѧʽ�� __________________��
��2��B����������ﻯѧʽΪ____________��C��Ԫ������Ϊ ___________________ ��
��3��D�ĵ�����ˮ��Ӧ�ķ���ʽΪ____________________________________________________��
���𰸡� Al2O3 P2O5 �� Cl2+H2O �� HCl+HClO
��������A��B��C��D��E���ֶ�����Ԫ�أ���λ��ͼ��֪��A�ڵڶ����ڣ�B��C��D���ڵ������ڣ���C��������Ϊx����A��������Ϊx-8��B��������Ϊx-1��D��������Ϊx+1��A��B��C��D����Ԫ��ԭ�Ӻ����56�����ӣ���x+��x-8��+��x-1��+��x+1��=56�����x=16����AΪO��BΪP��CΪS��DΪCl��1molE���������������ã��ڱ�״�����ܲ���33.6LH2�����������ʵ�����1.5mol����E�Ļ��ϼ�Ϊy�����ݵ���ת���غ㣺1mol��y=1.5mol��2�����y=3��E����������A�������Ӻ�����Ӳ�ṹ��ȫ��ͬ����EΪAl��
��1���������Ϸ�����֪A��E�γɵĻ�����Ļ�ѧʽ��Al2O3����2��P����������ﻯѧʽΪP2O5��C��Ԫ������Ϊ������3��D�ĵ���������ˮ��Ӧ�ķ���ʽΪCl2+H2O��HCl+HClO��

����Ŀ����������(NOCl,�۵�:-64.5 ��,�е�:-5.5 ��)��һ�ֻ�ɫ����,��ˮ��ˮ�⡣�����ںϳ���������ý�����м���ȡ�ʵ���ҿ���������һ�������ڳ��³�ѹ�ºϳɡ�
��1�������ͬѧ���Ʊ�ԭ����NO��Cl2,�Ʊ�װ������ͼ��ʾ:

Ϊ�Ʊ��������������,�±���ȱ�ٵ�ҩƷ��:
װ�â� | װ�â� | ||
��ƿ�� | ��Һ©���� | ||
�Ʊ�����Cl2 | MnO2 | ��___ | ��___ |
�Ʊ�����NO | Cu | ��___ | ��___ |
��2������ͬѧ���ü����Ƶõ�NO��Cl2�Ʊ�NOCl,װ����ͼ��ʾ:

��װ������˳��Ϊa��________(�������������ҷ���,��Сд��ĸ��ʾ)��
��װ�â��������ɽ�һ������NO��Cl2��,��һ��������____________��
��װ�â���������____________��
��װ�â�������β��ʱ,NOCl������Ӧ�Ļ�ѧ����ʽΪ________________��
��3������ͬѧ��������,�����ˮ��Ũ������Ũ����Ļ��ᣬһ�������¸û���������������Ⱥ������� �÷�Ӧ�Ļ�ѧ����ʽΪ__________________��
����Ŀ��Y��Z��W��R��M����Ԫ�أ�λ��Ԫ�����ڱ���ǰ�����ڣ����ǵĺ˵��������������������Ϣ��
Ԫ�� | �����Ϣ |
Y | ԭ�Ӻ�����6����ͬ�˶�״̬�ĵ��� |
Z | �ǽ���Ԫ�أ���̬ԭ�ӵ�s����ĵ���������p����ĵ���������ͬ |
W | ����Ԫ�أ���Zԭ�ӵļ۵�������ͬ |
R | �۲�����Ų�ʽΪ3d64s2 |
M | IB�壬�䱻������������ҵ�������� |
��ش��������⣨Y��Z��W��R��M������Ӧ��Ԫ�ط��ű�ʾ��:
��1��Z��WԪ����ȣ���һ�����ܽϴ����_____________________��M2+�ĺ�������Ų�ʽΪ________________________��
��2��M2Z���۵��M2W��_________������������������) �������ԭ��___________ ��
��3��N3-��YZ2�ǵȵ����壬��N3-�ĽṹʽΪ_________________ ��
��4��WZ2������Wԭ�Ӽ۲���Ӷ�����_____________�ԣ�WZ2��VSEPR ģ������Ϊ______________________��WZ3��̬Ϊ�����ӣ��÷�����Wԭ�ӵ��ӻ��������Ϊ__________________��WZ3�������廷״�ṹ��ͼ1��ʾ���ýṹ��Wԭ�ӵ��ӻ��������Ϊ__________���ýṹ��W-Z���������࣬һ�����Լ140pm����һ�����ԼΪ160pm���϶̵ļ�Ϊ___________(��ͼ2����ĸ) ���÷����к���___��������
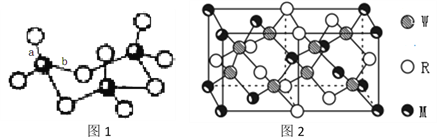
��5��MRW2�ľ�����ͼ2��ʾ����������a=0.524nm��c=1.032nm��MRW2�ľ�����ÿ��Mԭ����_______��Wԭ�������������ܶ���=_______g/cm3��ֻҪ������ʽ�����ؼ������ֵ�������ӵ�����ΪNA��6��02��1023mol-1����