题目内容
【题目】铜是重要金属,Cu的化合物在科学研究和工业生产中具有许多用途,如CuSO4溶液常用作电解液、电镀液等。请回答以下问题:
(1)CuSO4可由金属铜与浓硫酸反应制备,该反应的化学方程式为________________________________________________________________________。
(2)CuSO4粉末常用来检验一些有机物中的微量水分,其原因是________________________________________________________________________。
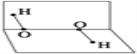
(3)SO![]() 中S以sp3杂化,SO
中S以sp3杂化,SO![]() 的立体构型是________。
的立体构型是________。
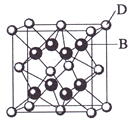
(4)元素金(Au)处于周期表中的第六周期,与Cu同族,金原子最外层电子排布式为____________。一种铜合金晶体具有立方最密堆积的结构,在晶胞中铜原子处于面心,金原子处于顶点位置,则该合金中铜原子与金原子数量之比为________;该晶体中,原子之间的作用力是________________________________________________________________________。
(5)CuSO4晶体的构成微粒是________和________,微粒间的作用力是________,该晶体属于________晶体。
(6)上述晶体具有储氢功能,氢原子可进入到由铜原子与金原子构成的四面体空隙中。若将铜原子与金原子等同看待,该晶体储氢后的晶胞结构与CaF2的结构相似,该晶体储氢后的化学式应为________。
【答案】 Cu+2H2SO4(浓)![]() CuSO4+SO2↑十+2H2O 白色CuSO4粉末和水结合生成蓝色的CuSO4·5H2O晶体 正四面体形 6s1 3∶1 金属键 Cu2+ SO
CuSO4+SO2↑十+2H2O 白色CuSO4粉末和水结合生成蓝色的CuSO4·5H2O晶体 正四面体形 6s1 3∶1 金属键 Cu2+ SO![]() 离子键 离子 Cu3AuH8
离子键 离子 Cu3AuH8
【解析】(1)Cu和浓硫酸在加热条件下反应生成二氧化硫和硫酸铜,反应的方程式为Cu+2H2SO4(浓)![]() CuSO4+SO2↑十+2H2O;(2)(2)CuSO4粉末常用来检验一些有机物中的微量水分,其原因是白色CuSO4粉末和水结合生成蓝色的CuSO4·5H2O晶体;(3)硫酸根中心原子的价层电子对为:孤对电子数
CuSO4+SO2↑十+2H2O;(2)(2)CuSO4粉末常用来检验一些有机物中的微量水分,其原因是白色CuSO4粉末和水结合生成蓝色的CuSO4·5H2O晶体;(3)硫酸根中心原子的价层电子对为:孤对电子数![]() =0,成键电子对数4,所以为正四面体结构;(4)元素金(Au)处于周期表中的第六周期,与Cu同族,则最外层电子数为1,则最外层电子排布式为6s1,在晶胞中Cu原子处于面心,N(Cu)=6×
=0,成键电子对数4,所以为正四面体结构;(4)元素金(Au)处于周期表中的第六周期,与Cu同族,则最外层电子数为1,则最外层电子排布式为6s1,在晶胞中Cu原子处于面心,N(Cu)=6×![]() =3,Au原子处于顶点位置,N(Au)=8×
=3,Au原子处于顶点位置,N(Au)=8×![]() =1,则该合金中Cu原子与Au原子数量之比为3:1,为金属晶体,原子间的作用力为金属键键合力;(5)CuSO4晶体的构成微粒是Cu2+和SO42-,微粒间的作用力是离子键,该晶体属于离子晶体;(6)CaF2的结构如图
=1,则该合金中Cu原子与Au原子数量之比为3:1,为金属晶体,原子间的作用力为金属键键合力;(5)CuSO4晶体的构成微粒是Cu2+和SO42-,微粒间的作用力是离子键,该晶体属于离子晶体;(6)CaF2的结构如图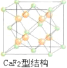 ,氢原子可进入到由Cu原子与Au原子构成的四面体空隙中,则H原子应位于晶胞内部,则应含有8个H,则化学式为Cu3AuH8。
,氢原子可进入到由Cu原子与Au原子构成的四面体空隙中,则H原子应位于晶胞内部,则应含有8个H,则化学式为Cu3AuH8。

【题目】某化学小组在实验室制取 Na2O2。查阅资料可知:钠与空气在 453473K 之间可生成 Na2O,迅速提高温度到 573673K 之间可生成 Na2O2,若温度提高到 733873K 之间 Na2O2 可分解。
(1)甲组设计制取 Na2O2 装置如图1。
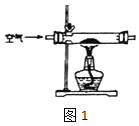
①使用该装置制取的 Na2O2 中可能含有的杂质为_____。.
A.NaCl B.Na2CO3 C.Na2O D.NaOH E.NaHCO3
②该小组为测定制得的 Na2O2 样品的纯度,设计可能用到的装置如图 2:烧瓶中发生的主要反应的化学方程式是_________。测定装置的接口从 左至右正确的连接顺序是_____。
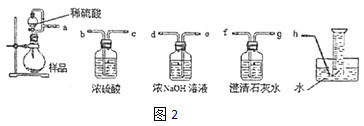
(2)乙组从反应历程上分析该测定反应存在中间产物,从而导致测定结果_____ (填“偏大”或“偏 小”)。为证明其分析的正确性,设计实验方案如下:
实验方案 | 产生的现象 |
Ⅰ.取烧瓶中的反应液加入少量 MnO2 粉末 | 有大量气泡逸出 |
Ⅱ.向 NaOH 稀溶液中加入 23 滴酚酞试液,然后加入少量的反应液 | 溶液先变红后褪色开始无明显现象。 |
Ⅲ.向反应液中加入 23 滴酚酞试液,充分振荡,然后逐滴加入 过量的 NaOH 稀溶液 | 加 NaOH 溶液先 变红后褪色 |
在上述实验中,能够证明乙组分析正确的最佳方案是_________ (填实验序号)。_______________组实验得不出结论,原因是_____。
(3)丙组根据上述提供的有关信息,设计一个方案可准确的测定样品的纯度。请简述实验操作和需 要测定的有关数据__________________________________