��Ŀ����
1���л���A��B��C��D���������ʣ���1������֧���Ļ�����A�ķ���ʽΪC4H6O2��A����ʹBr2�����Ȼ�̼��Һ��ɫ��1molA��1molNaHCO3����ȫ��Ӧ����A�Ľṹ��ʽ��
 ��д����A������ͬ�����ŵ�A������ͬ���칹��Ľṹ��ʽCH2=CH-CH2-COOH��CH3-CH=CH-COOH��
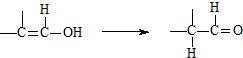
��д����A������ͬ�����ŵ�A������ͬ���칹��Ľṹ��ʽCH2=CH-CH2-COOH��CH3-CH=CH-COOH����2��������B��C��H��O����Ԫ�أ�������Ϊ60������̼����������Ϊ60%�������������Ϊ13.33%��B�ڴ���Cu�������±�����������C��C�ܷ���������Ӧ����B�Ľṹ��ʽ��CH3CH2CH2OH��д��C����������Ӧ�Ļ�ѧ����ʽ��CH3CH2CHO+2Ag��NH3��2OH$\stackrel{��}{��}$CH3CH2COONH4+3NH3+2Ag��+H2O��
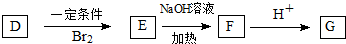
��3��D��NaOHˮ��Һ�м��ȷ�Ӧ��������A�����κ�B����Ӧ��Ӧ�Ļ�ѧ����ʽ��
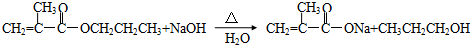 ��
��
���� ��1��A�ķ���ʽΪC4H6O2��1mol A��1mol NaHCO3����ȫ��Ӧ��A���Ӻ���1��-COOH��A����ʹBr2�����Ȼ�̼��Һ��ɫ��A�IJ����Ͷ�Ϊ$\frac{2��4+2-6}{2}$=2���ʻ�����1��̼̼˫������A����֧������AΪ ��
��
��2��������B����C��H��O����Ԫ�أ�������Ϊ60��̼����������Ϊ60%�������������Ϊ13.33%���������N��C��=$\frac{60��60%}{12}$=3��N��H��=$\frac{60��13.33%}{1}$=8��N��O��=$\frac{60-12��3-8}{16}$=1����B�ķ���ʽΪC3H8O��B�ڴ���Cu�������±�������C��C�ܷ���������Ӧ����BΪCH3CH2CH2OH��CΪCH3CH2CHO��
��3��D��NaOHˮ��Һ�м��ȷ�Ӧ��������A�����κ�B����DΪCH2=C��CH3��COOCH2CH2CH3��
��� �⣺��1��A�ķ���ʽΪC4H6O2��1mol A��1mol NaHCO3����ȫ��Ӧ��A���Ӻ���1��-COOH��A����ʹBr2�����Ȼ�̼��Һ��ɫ��A�IJ����Ͷ�Ϊ$\frac{2��4+2-6}{2}$=2���ʻ�����1��̼̼˫������A����֧������AΪ ����A������ͬ�����ŵ�A������ͬ���칹��Ľṹ��ʽΪ��CH2=CH-CH2-COOH CH3-CH=CH-COOH��
����A������ͬ�����ŵ�A������ͬ���칹��Ľṹ��ʽΪ��CH2=CH-CH2-COOH CH3-CH=CH-COOH��
�ʴ�Ϊ�� ��CH2=CH-CH2-COOH��CH3-CH=CH-COOH��
��CH2=CH-CH2-COOH��CH3-CH=CH-COOH��
��2��������B����C��H��O����Ԫ�أ�������Ϊ60��̼����������Ϊ60%�������������Ϊ13.33%���������N��C��=$\frac{60��60%}{12}$=3��N��H��=$\frac{60��13.33%}{1}$=8��N��O��=$\frac{60-12��3-8}{16}$=1����B�ķ���ʽΪC3H8O��B�ڴ���Cu�������±�������C��C�ܷ���������Ӧ����BΪCH3CH2CH2OH��CΪCH3CH2CHO��
CΪCH3CH2CHO������������Ӧ�ķ���ʽΪCH3CH2CHO+2Ag��NH3��2OH$\stackrel{��}{��}$CH3CH2COONH4+3NH3+2Ag��+H2O��
�ʴ�Ϊ��CH3CH2CH2OH��CH3CH2CHO+2Ag��NH3��2OH$\stackrel{��}{��}$CH3CH2COONH4+3NH3+2Ag��+H2O��
D��NaOHˮ��Һ�м��ȷ�Ӧ��������A�����κ�B����DΪCH2=C��CH3��COOCH2CH2CH3���÷�Ӧ����ʽΪ��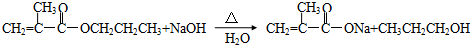 ��
��
�ʴ�Ϊ��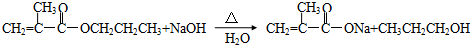 ��
��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬������ѧ���ķ��������������Ŀ��飬�ѶȲ�����ȷ��B�ķ���ʽ�����ݷ����ķ�Ӧ�жϺ��еĹ����ţ�ȷ���л���A��B�Ľṹ����Ҫѧ���������չ����ŵ�������ת����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| Z | W | ||
| X | Y |
| A�� | XԪ����������ﻯѧʽΪX2O3 | |
| B�� | ԭ�Ӱ뾶��X��Y��Z��W | |
| C�� | �����̬�⻯������ȶ��ԣ�W��Y | |
| D�� | YԪ�ص���������������NaOH��Һ��Ӧ��Ҳ����HF��Һ��Ӧ���������������� |
| A�� | ���Ӱ뾶��S2-��O2-��Na+��Al3+ | B�� | ���ȶ��ԣ�HCl��H2S��PH3��AsH3 | ||
| C�� | �縺�ԣ�F��O��N��C | D�� | ��һ�����ܣ�F��O��N��C |
| A�� | �� | B�� | ���� | C�� | ��ϩ | D�� | �Ȼ��� |
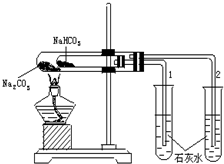 ʵ����������ͼ��ʾװ�ý���Na2CO3��NaHCO3���ȶ��ԶԱ�ʵ�飮��ش�
ʵ����������ͼ��ʾװ�ý���Na2CO3��NaHCO3���ȶ��ԶԱ�ʵ�飮��ش�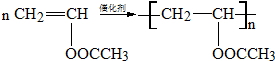 ��
��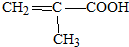 ��
��
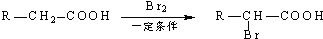
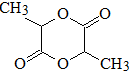 ��д��N������ȥ��Ӧ�Ļ�ѧ����ʽ��CH3CH��OH��COOH$��_{��}^{Ũ����}$CH2=CHCOOH+H2O��
��д��N������ȥ��Ӧ�Ļ�ѧ����ʽ��CH3CH��OH��COOH$��_{��}^{Ũ����}$CH2=CHCOOH+H2O�� Ϊԭ�ϣ��������Լ���ѡ�����ϳ�
Ϊԭ�ϣ��������Լ���ѡ�����ϳ�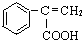 ������ͼ���£���ش�
������ͼ���£���ش�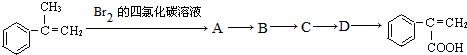
 ��
�� ��
�� ij�¶�ʱ��2L������X��Y��Z������̬���ʵ����ʵ�����n����ʱ�䣨t���仯��������ͼ��ʾ����ͼ�����ݷ�����
ij�¶�ʱ��2L������X��Y��Z������̬���ʵ����ʵ�����n����ʱ�䣨t���仯��������ͼ��ʾ����ͼ�����ݷ�����