��Ŀ����
5��CO2�뻯�����Ӧ���ɻ�������뻯�����Ӧ���ɻ���������練Ӧ�ٺ͢���ʾ�������Լ������P��Ӧ������ʡ�ԣ���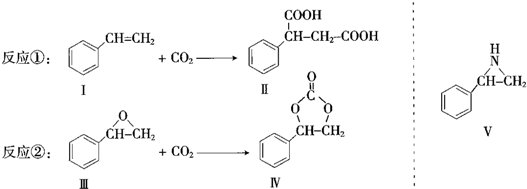
��1���������ķ���ʽΪC8H8O��1mol��������ȫȼ��������9.5mol O2��
��2���Ի�����IΪԭ�ϵõ�һ�ֹ㷺Ӧ�õ����ϣ��ýṹ��ʽ��ʾ�����ϵ����
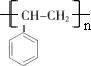 ��
����3��������V����������ƣ�д��������V��CO2 ��Ӧ�IJ���Ľṹ��ʽ��
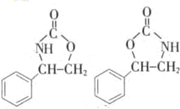 ����дһ�֣���
����дһ�֣�����4�����й��ڢ�͢���˵����ȷ����BD����˫ѡ������ĸ����
A�������ڷ����� B��������NaOH��Ӧ
C��������Na��Ӧ�ų�H2 D.1mol II����������3mol H2�����ӳɷ�Ӧ��
���� ��1���ɽṹ��ʽ��ȷ������ʽ�����ͨʽ��x+$\frac{y}{4}$-$\frac{z}{2}$�������������
��2��������I����̼̼˫�����ɷ����Ӿ۷�Ӧ���ɸ߾��
��3��������V��CO2 ��Ӧ�IJ����к����ļ���
��4��II�����Ȼ����������ԣ��������������ɷ���ˮ�ⷴӦ��ֻ�б��������������ӳɷ�Ӧ��
��� �⣺��1���ɽṹ��ʽ��֪��ķ���ʽΪC8H8O��1mol���л���ȼ��ʱ�������������ʵ���Ϊ��8+$\frac{8}{4}$-$\frac{1}{2}$��mol=9.5mol��
�ʴ�Ϊ��C8H8O��9.5��
��2��������I����̼̼˫�����ɷ����Ӿ۷�Ӧ���ɱ���ϩ���ṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3��������V����CO2�������Ʒ�Ӧ�ڵķ�Ӧ����ԭ���ǣ�O=C=O�е�һ��̼�����Ͽ����л���Ͽ�̼�����ӳɣ������ּӳɷ�ʽ������Ϊ ��
��
�ʴ�Ϊ�� ����дһ�֣���
����дһ�֣���
��4��A��������OԪ�أ������ڷ���������A����
B��II�����Ȼ����������ԣ��������������ɷ���ˮ�ⷴӦ��������NaOH��Ӧ����B��ȷ��
C���������ǻ�����Na����Ӧ����C����
D��ֻ�б��������������ӳɷ�Ӧ����1mol II����������3mol H2�����ӳɷ�Ӧ����D��ȷ��
�ʴ�Ϊ��BD��
���� ���⿼���л�����ƶϼ��ṹ�����ʣ�Ϊ��Ƶ���㣬���չ����������ʵĹ�ϵΪ���Ĺؼ������ط������ƶ�������Ǩ��Ӧ�������Ŀ��飬��Ŀ�ѶȲ���

| A�� | ��ѿ�Ǽ���ˮ�������ܷ���������Ӧ | |
| B�� | ������ˮ������ղ����Ƕ��� | |
| C�� | ��ϩ�ͱ�����ʹ��ˮ��ɫ����ɫ��ԭ����ͬ | |
| D�� | ʯ���ѽ����֬���������ɸ߷�������С���ӵĹ��� |

| A�� | 3��1 | B�� | 4��5 | C�� | 2��1 | D�� | 3��2 |
| A�� | ԭ�Ӱ뾶��x��y��z | B�� | �縺�ԣ�x��z��y | ||
| C�� | ԭ��������y��z��x | D�� | ��һ�����ܣ�z��y��x |
 ����1Ħ����˹ƥ�ֺ�����Na��H��Һ��ַ�Ӧ������NaOH�����ʵ���Ϊ��������
����1Ħ����˹ƥ�ֺ�����Na��H��Һ��ַ�Ӧ������NaOH�����ʵ���Ϊ��������| A�� | 1 mol | B�� | 2 mol | C�� | 3 mol | D�� | 4 mol |
| A�� | C2H6��C2H4 | B�� | CH3CH2Cl��CH3CH2CH2CCl3 | ||
| C�� | CH3CH2CH3��C5H12 | D�� | CH3CH2CH2OH��HOCH2CH2OH |
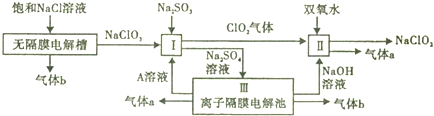
 ���е�196.5�森��ش��������⣺
���е�196.5�森��ش��������⣺ �ķе㣾196.5�森�����������������=����ԭ����
�ķе㣾196.5�森�����������������=����ԭ����