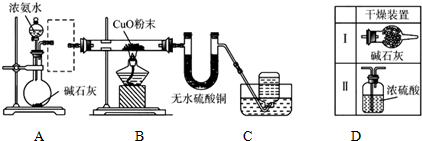
��Ŀ����
20����֪�л���ס��ҡ�����������Ϣ��| �� | �� | �� | |
| ����Ԫ�� | C��H | C��H��F | C��H��F |
| ���������� | 26 | ||
| �ṹ�ص� | �����л��� | ||
��1���ķ���ʽΪC3H8������������2����ԭ�ӱ�Fԭ��ȡ�������õ��л����������4�֣�
��2���������������Ļ�������ɴ���ijЩ�ƻ�������ķ�������Ʒ������������������C��H��F��ԭ�Ӹ���֮��Ϊ1��2��2�����ҵĽṹʽΪ
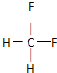 �����й����ҵ�������ȷ����D��
�����й����ҵ�������ȷ����D��A������ӹ���Ϊ���������� B������ʹ��ˮ��ɫ
C��1mol ���������1mol F2 ����ȡ����Ӧ D����û��ͬ���칹��
��3�����ס��Ұ����ʵ���֮��1��1��ϣ����û�����ƽ��Ħ���������ڱ���Ħ���������ұ����Ӳ�����ͬ���칹�壬����ķ���ʽΪC2H5F��
���� ��1���������ֻ��C��H����Ԫ�أ�Ϊ�����л��ӦΪ����������ʽ����CnH2n+2�������к���26�����ӣ�����6n+2n+2=26��n=3�����ΪC3H8��
��2���ҷ�����C��H��Fԭ�Ӹ�����Ϊl��2��2�������к���26�����ӣ�ӦΪCH2F2��������Ϊ26�����ϣ���ϵ����Ŀռ�ṹ�ж�CH2F2�Ľṹ�ص㼰���е����ʣ�
��3�����ס��Ұ����ʵ���֮��1��1������û�����ƽ��Ħ���������ڱ���Ħ��������������Է�������Ϊ��$\frac{44+52}{2}$=48���ҷ����к���26�����ӣ�ӦΪC2H5F��
��� �⣺��1���������ֻ��C��H����Ԫ�أ���Ϊ�����л�����ӦΪ����������ʽ����CnH2n+2�������к���26�����ӣ�����6n+2n+2=26��n=3�����ΪC3H8����������������Hԭ�ӱ�Fԭ�Ӵ��棬���ò��������CH3CH2CHF2��CH3CF2CH3��CH2FCHFCH3��CH2FCH2CH2F��4�֣�
�ʴ�Ϊ��C3H8��4��
��2���ҷ�����C��H��Fԭ�Ӹ�����Ϊl��2��2�������к���26�����ӣ������ʽΪCH2F2��CH2F2Ϊ���ۻ���������ʽΪ��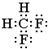 �������й��õ��ӶԻ��ɶ���Ϊ�ṹʽ������ṹʽΪ��
�������й��õ��ӶԻ��ɶ���Ϊ�ṹʽ������ṹʽΪ��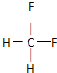 ��
��
A������Ϊ��������ṹ����CH2F2Ϊ�������νṹ�������������壬��A����
B��������в����ڲ����ͼ�������ʹ��ˮ��ɫ����B����
C��1mol���к���2molHԭ�ӣ��������2mol F2 ����ȡ����Ӧ����C����
D������������H����Ч����CH2F2û��ͬ���칹�壬��D��ȷ��
�ʴ�Ϊ��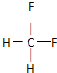 ��D��
��D��
��3�����ס��Ұ����ʵ���֮��1��1������û�����ƽ��Ħ���������ڱ���Ħ��������������Է�������Ϊ��$\frac{44+52}{2}$=48���ҷ����к���26�����ӣ�ӦΪC2H5F��
�ʴ�Ϊ��C2H5F��
���� ���⿼���л������ʽ���ṹ��ʽ��ȷ������Ŀ�Ѷ��еȣ���ȷ������ͨʽ�Լ�ԭ�ӵĹ���Ϊ������Ĺؼ�������������ѧ���ķ������������������Ӧ�û���֪ʶ��������

| A�� | ��ϩ�� | B�� | �������� | C�� | �ױ� | D�� | ���� |
| A�� | 1mol Na2CO3��������1Lˮ�������Һ�������ʵ���Ũ��Ϊ1mol/L | |
| B�� | 40g NaOH��������100gˮ����ɵ���Һ�����ٷֱ�Ũ��Ϊ40% | |
| C�� | 22.4L��������ˮ���1L��Һ�������ʵ���Ũ��Ϊ1mol/L | |
| D�� | 1mol NaCl��������ˮ���1L��Һ�������ʵ���Ũ��Ϊ1mol/L |
| A�� | Na��Mg��ˮ��Ӧ�������������Fe����һ��������ˮ��ӦҲ���ɼ������ | |
| B�� | CaC2��ˮ�⣺CaC2+2H2O�TCa��OH��2+C2H2������Al4C3Ҳ��ˮ�⣺Al4C3+12H2O�T4Al��OH��3��+3CH4�� | |
| C�� | Fe���û�����ͭ��Һ��ͭ����NaҲ���û�����ͭ��Һ��ͭ | |
| D�� | ��ҵ�ϵ������MgCl2����ȡ����þ����Ҳ�����õ������AlCl3����ȡ������ |
| A�� | ���ϵ����⻯����۷е������� | |
| B�� | ���ϵ��µ��ʵĻ�ԭ������ | |
| C�� | ���ϵ�������������ˮ������������ | |
| D�� | ���ϵ���ԭ�ӵõ��ӵ���������ǿ |
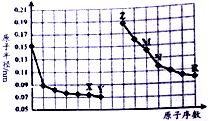
| A�� | Y��R����Ԫ�ص���̬�⻯���ȶ��Դ�С��Y��R | |
| B�� | �����ӵİ뾶��X��Z��M | |
| C�� | ��X��N����Ԫ����ɵĻ����ﲻ�����κ��ᷴӦ��������ǿ�Ӧ | |
| D�� | Z�����ܴ�M��RԪ�ع��ɵ�����Һ���û�������M |
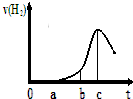 ij��ȤС����С����ý������ᷴӦ����ʵ�飬��5.4g����ƬͶ��500mL 0.5mol•L-1��������Һ�У���ͼΪ��Ӧ�������������뷴Ӧʱ��Ĺ�ϵͼ��
ij��ȤС����С����ý������ᷴӦ����ʵ�飬��5.4g����ƬͶ��500mL 0.5mol•L-1��������Һ�У���ͼΪ��Ӧ�������������뷴Ӧʱ��Ĺ�ϵͼ��