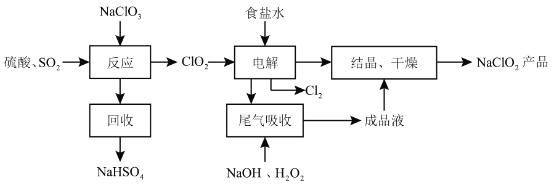
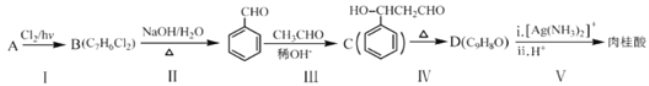
��Ŀ����
����Ŀ��ʵ����Ҫ��CuSO4��5H2O��������500 mL 0.1 mol��L��1 CuSO4��Һ���ش��������⣺
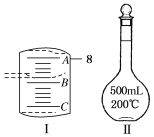
(1)Ӧ����������ƽ��ȡCuSO4��5H2O________g��
(2)��ͼ���ʾ10 mL��Ͳ��Һ���λ�ã�A��B��B��C�̶ȼ����1 mL������̶�AΪ8����Ͳ��Һ��������________mL��
(3)��ʵ������ͼ����ʾ�������������������������Һ��Ũ���к�Ӱ�죿(����ƫ������ƫ����������Ӱ����)
A������ǰ����ƿ�ײ���ˮ��__________________________��
B������ʱ��ˮ�����̶���____________________________��
C�����ն���ʱ���ӹ۲�Һ��__________________________��
���𰸡�12.5 7.2 ��Ӱ�� ƫ�� ƫ��
��������
����m=CVM������Ҫ���ʵ���������ͲС�̶������£���̶������ϣ�����Ͳ��Һֻ�ܼ�¼��С�����1λ)������C=![]() �����������������������������ʵ����ʵ�������Һ�������Ӱ�졣
�����������������������������ʵ����ʵ�������Һ�������Ӱ�졣
��1��ʵ�����õ�����CuSO45H2O������500mL 0.1molL-1CuSO4��Һ��Ӧ����������ƽ��ȡ����������m=0.1molL-1��0.5L��250g/mol=12.5g����2����ͲС�̶������£���̶������ϣ�����A�̶�Ϊ8�������B�̶�Ϊ7��C�̶�Ϊ6����B��A����5���̶ֿȣ�ÿ���̶ֿ�Ϊ0.2 mL����Һ��ʾ��Ϊ7.2mL����3������ǰ����ƿ�ײ���ˮ�飬�����ʵ����ʵ���û��Ӱ�죬���Բ�Ӱ�����ƽ��������ʱ��ˮ�����̶��ߣ��������Ƶ���Һ���ƫ����C=![]() ��֪���Ƶ���ҺŨ��ƫ�ͣ����ն���ʱ���ӹ۲�Һ�棬���¼��������ˮ���ƫС������C=
��֪���Ƶ���ҺŨ��ƫ�ͣ����ն���ʱ���ӹ۲�Һ�棬���¼��������ˮ���ƫС������C=![]() ��֪���Ƶ���ҺŨ��ƫ�ߡ�
��֪���Ƶ���ҺŨ��ƫ�ߡ�