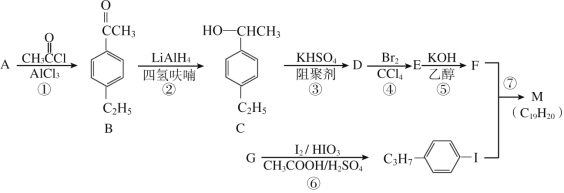
ΧβΡΩΡΎ»ί
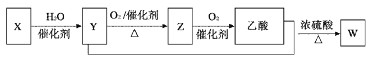
ΓΨΧβΡΩΓΩΝρ «“Μ÷÷ΑκΒΦΧε≤ΡΝœΘ§ τ”ΎœΓ…ΔΫπ τΘ§±Μ”ΰΈΣΓΑœ÷¥ζΙΛ“ΒΓΔΙζΖά”κΦβΕΥΦΦ θΒΡΈ§…ζΥΊΘ§¥¥‘λ»ΥΦδΤφΦΘΒΡ«≈ΝΚΓ±Θ§ «Β±¥ζΗΏΦΦ θ–¬≤ΡΝœΒΡ÷ß≥≈≤ΡΝœΓΘ“‘μΎΜ·―«Ά≠‘ϋΘ®÷ς“ΣΈοœύΈΣCu2TeΓΔCuΓΔCuSO45H2OΓΔAuΓΔAgΒ»Θ©ΈΣ‘≠ΝœΧα»Γ”κ÷Τ±ΗTeO2ΚΆΒΞ÷ TeΒΡΙΛ“’Νς≥Χ»γΆΦΥυ ΨΘΚ
“―÷ΣΘΚΓΑΥ°ΫβΓ±Ζ¥”ΠΈΣH2TeO3(―«μΎΥα)=TeO2Γΐ+H2OΓΘ
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©Cu2Te÷–TeΒΡΜ·ΚœΦέΈΣ___ΓΘ
Θ®2Θ©ΓΑΥαΫΰΓ± ±Θ§“Σ Ι6molCu»ήΫβΘ§”κCuΖ¥”ΠΒΡNaC1O3ΒΡΈο÷ ΒΡΝΩΈΣ___ΓΘ
Θ®3Θ©–¥≥ωΓΑΥαΫΰΓ± ±Cu2TeΖΔ…ζΉΣΜ·ΒΡάκΉ”ΖΫ≥Χ ΫΘΚ___ΓΘ
Θ®4Θ©»ΓμΎΜ·―«Ά≠‘ϋ100gΘ§¬»ΥαΡΤΧμΦ”÷ ΝΩΚΆΝρΥα≈®Ε»Ε‘μΎΜ·―«Ά≠‘ϋΫΰ≥ω–ßΙϊΒΡ”Αœλ»γΆΦΥυ ΨΘΚ
―Γ‘ώΉνΦ―ΒΡ¬»ΥαΡΤΧμΦ”÷ ΝΩΈΣ___gΘ§―Γ‘ώΝρΥαΒΡ≈®Ε»‘ΦΈΣ___mol/L(±ΘΝτ–Γ ΐΒψΚσ“ΜΈΜΘ©ΓΘ
Θ®5Θ©ΓΑΦνΫΰ‘ϋΓ±÷–Κ§”–ΒΡΫπ τΒΞ÷ ÷ς“Σ”–___(ΧνΜ·―ß ΫΘ©Θ§ΨΏ”–ΚήΗΏΒΡΨ≠ΦΟάϊ”ΟΦέ÷ΒΓΘ
Θ®6Θ©ΓΑΦνΫΰ“ΚΓ±άϊ”ΟΝρΥαΒςΫΎ»ή“ΚpH÷Ν5.5Θ§≥ΝΒμ≥ωTeO2Θ§ΗΟΙΐ≥ΧΒΡάκΉ”ΖΫ≥Χ ΫΈΣ____ΓΘ
Θ®7Θ©Βγ≥ΝΜΐΖ® «ΙΛ“Β÷–÷Τ±Η¥ΩTeΒΡ≥Θ”ΟΖΫΖ®Θ§“‘≤Μ–βΗ÷ΑεΚΆΤ’Ά®ΧζΑεΉς“θΓΔ―τΦΪΘ§‘Ύ“ΜΕ®ΒΡΒγΝςΟήΕ»ΓΔΈ¬Ε»œ¬ΒγΫβΦνΫΰ“ΚΘ§μΎ‘ΣΥΊ“‘Ϋπ τTe–Έ Ϋ‘Ύ“θΦΪΈω≥ωΘ§‘ρ“θΦΪΒΡΒγΦΪΖ¥”Π ΫΈΣ___ΓΘ
ΓΨ¥πΑΗΓΩ-2 2mol Cu2Te+4C1O3-+12H+=6Cu2++3H2TeO3+4Cl-+3H2O 50 0.7 AuΓΔAg TeO32-+2H+= TeO2Γΐ+ H2O TeO32-+ 3H2O+ 4e-=Te+6OH-
ΓΨΫβΈωΓΩ
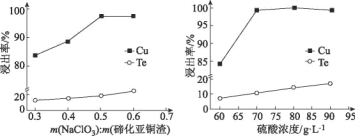
μΎΜ·―«Ά≠‘ϋΘ®÷ς“ΣΈοœύΈΣCu2TeΓΔCuΓΔCuSO45H2OΓΔAuΓΔAgΒ»Θ©Φ”»κΉψΝΩΝρΥα»ήΫβΘ§Τδ÷–CuΓΔAuΓΔAg≤Μ”κΝρΥαΖ¥”ΠΘ§–Έ≥…Κ§”–Cu+ΓΔCu2+ΓΔTe2+ΓΔSO42-ΒΡ»ή“ΚΘ§‘ΌΦ”»κ¬»ΥαΡΤΘ§‘ΎΥα–‘ΧθΦΰœ¬¬»ΥαΗυάκΉ”ΨΏ”–«Ω―θΜ·–‘Θ§ΫΪ»ή“Κ÷–CuΓΔCu+―θΜ·ΈΣCu2+Θ§Te2+±Μ―θΜ·ΉΣΜ·ΈΣH2TeO3Θ§Ψ≠ΙΐΥ°ΫβH2TeO3ΉΣΜ·ΈΣTeO2Θ§Ιΐ¬ΥΚσΥαΫΰ¬Υ“Κ÷–Κ§”–Cu2+ΓΔCl-Θ§Υα¬Υ‘ϋΒΡ≥…Ζ÷ΈΣAuΓΔAgΓΔTeO2Θ§œρΥα¬Υ‘ϋ÷–Φ”»κ«β―θΜ·ΡΤΘ§ΫΪTeO2»ήΫβΉΣΜ·Na2TeO3Θ§‘ΎΙΐ¬ΥΘ§ΒΟΒΫΦνΫΰ‘ϋΚΆΦνΫΰ“ΚΘ§ΦνΫΰ‘ϋ÷–÷ς“ΣΚ§”–AuΓΔAgΘ§ΦνΫΰ“Κ÷ς“ΣΈΣNa2TeO3Θ§Ε‘ΦνΫΰ“ΚΒγΫβΩ…ΒΟΒΫTeΘ§œρΦνΫΰ“Κ÷–Φ”»κΝρΥαΒςΫΎpH÷Β…ζ≥…TeO2Θ§Ψί¥ΥΖ÷ΈωΫβ¥πΓΘ
(1)μΎΜ·―«Ά≠Cu2Te÷–Cu ΈΣ+1ΦέΘ§TeΜ·ΚœΦέΈΣ-2ΦέΘΜ
(2) 6molCuΉΣΜ·ΈΣCu2+ ß»Ξ12mole-Θ§1mol NaC1O3ΉΣΜ·ΈΣCl-ΒΟΒΫ6mol e-Θ§Ι “Σ Ι6molCu»ήΫβΘ§”κCuΖ¥”ΠΒΡNaC1O3ΒΡΈο÷ ΒΡΝΩΈΣ2molΘΜ
(3)–¥≥ωΓΑΥαΫΰΓ± ±Θ§‘ΎΥα–‘ΧθΦΰœ¬¬»ΥαΗυάκΉ”ΨΏ”–«Ω―θΜ·–‘Θ§ Te2+±Μ―θΜ·ΉΣΜ·ΈΣH2TeO3Θ§Cu2TeΖΔ…ζΉΣΜ·ΒΡάκΉ”ΖΫ≥Χ ΫΘΚCu2Te+4C1O3-+12H+=6Cu2++3H2TeO3+4Cl-+3H2OΘΜ
(4)Ά≠ΚΆμΎΒΡΫΰ≥ω¬ Υφ¬»ΥαΡΤΧμΦ”÷ ΝΩΒΡ‘ωΦ”Εχ‘ω¥σΘ§Β±m(NaC1O3): m(μΎΜ·―«Ά≠‘ϋ)ΘΨ0.5 ±Θ§Ά≠Ϋΰ≥ω¬ ±δΜ·≤Μ¥σΘ§ΕχμΎΒΡΫΰ≥ω¬ ¥σΖυΕ»‘ωΦ”Θ§“ρ¥ΥΘ§ΈΣΫΒΒΆμΎΒΟΥπ ßΦΑΆ≠ΒΡ”––ßΖ÷άκΘ§―Γ‘ώm(NaC1O3): m(μΎΜ·―«Ά≠‘ϋ)=0.5Θ§μΎΜ·―«Ά≠‘ϋΈΣ100gΘ§Ι NaC1O3ΧμΦ”÷ ΝΩΈΣ50gΘΜΆ≠ΚΆμΎΒΡΫΰ≥ω¬ ΥφΉ≈ΝρΥα≈®Ε»ΒΡ‘ω¥σΕχ…ΐΗΏΘ§Β±ΝρΥα≈®Ε»‘ω÷Ν70g/L ±Θ§ΦΧ–χ‘ωΦ”ΝρΥα≈®Ε»Ε‘Ά≠ΒΡΫΰ≥ω¬ ”Αœλ≤Μ¥σΘ§ΒΪΜαœ‘÷χ¥ΌΫχμΎΒΡ»ήΫβΘ§“ρ¥ΥΘ§―Γ‘ώΝρΥα÷ ΝΩ≈®Ε»ΈΣ70g/LΘ§Έο÷ ΒΡΝΩ≈®Ε»‘ΦΈΣ![]() Γ÷0.7mol/LΘΜ
Γ÷0.7mol/LΘΜ
(5)ΗυΨίΖ÷ΈωΘ§AuΓΔAg–‘÷ Έ»Ε®Θ§≤Μ”κΝρΥαΓΔ«β―θΜ·ΡΤ»ή“ΚΖ¥”ΠΘ§Ι ΓΑΦνΫΰ‘ϋΓ±÷–Κ§”–ΒΡΫπ τΒΞ÷ ÷ς“Σ”–AuΓΔAgΘΜ
(6)ΗυΨίΖ÷ΈωΘ§ΦνΫΰ“Κ÷ς“ΣΈΣNa2TeO3Ά®ΙΐΝρΥαΒςΫΎ»ή“ΚpHΙΐ≥Χ÷–ΒΡάκΉ”Ζ¥”ΠΖΫ≥Χ ΫΈΣTeO32-+2H+= TeO2Γΐ+ H2OΘΜ
(7)ΒγΫβΦνΫΰ“ΚΘ§μΎ‘ΣΥΊ“‘Ϋπ τTe–Έ Ϋ‘Ύ“θΦΪΈω≥ωΘ§‘ρ“θΦΪΒΡΒγΦΪΖ¥”Π ΫΈΣTeO32-+3H2O+ 4e-=Te+6OH-ΓΘ

 Οϊ–ΘΩΈΧΟœΒΝ–¥πΑΗ
Οϊ–ΘΩΈΧΟœΒΝ–¥πΑΗΓΨΧβΡΩΓΩΧΦΚΆΒΣΒΡΜ·ΚœΈο‘Ύ…ζ≤ζ…ζΜν÷–ΙψΖΚ¥φ‘ΎΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©»ΐ¬»Μ·ΒΣΘ®NCl3Θ© «“Μ÷÷ΜΤ…ΪΓΔ”ΆΉ¥ΓΔΨΏ”–¥ΧΦΛ–‘ΤχΈΕΒΡΜ”ΖΔ–‘”–ΕΨ“ΚΧεΘ§Τδ‘≠Ή”Ψυ¬ζΉψ8eΘ≠ΫαΙΙΓΘ–¥≥ωΤδΒγΉ” Ϋ_________ΓΘ¬»ΦνΙΛ“Β…ζ≤ζ ±Θ§”…”Ύ ≥―ΈΥ°÷–Ά®≥ΘΚ§”–…ΌΝΩNH4ClΘ§Εχ‘Ύ“θΦΪ«χ”κ…ζ≥…ΒΡ¬»ΤχΖ¥”Π≤ζ…ζ…ΌΝΩ»ΐ¬»Μ·ΒΣΘ§ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ_______ΓΘ
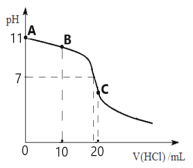
Θ®2Θ©“ΜΕ®ΧθΦΰœ¬Θ§≤ΜΆ§Έο÷ ΒΡΝΩΒΡCO2”κ≤ΜΆ§ΧεΜΐΒΡ1.0 mol/L NaOH»ή“Κ≥δΖ÷Ζ¥”ΠΖ≈≥ωΒΡ»»ΝΩ»γœ¬±μΥυ ΨΘΚ
Ζ¥”Π –ρΚ≈ | CO2ΒΡΈο÷ ΒΡΝΩ/mol | NaOH»ή“ΚΒΡΧεΜΐ/L | Ζ≈≥ωΒΡ»»ΝΩ /kJ |
1 | 0.5 | 0.75 | a |
2 | 1.0 | 2.00 | b |
ΗΟΧθΦΰœ¬CO2”κNaOH»ή“ΚΖ¥”Π…ζ≥…NaHCO3ΒΡ»»Μ·―ßΖ¥”ΠΖΫ≥Χ ΫΈΣΘΚ_______ΓΘ
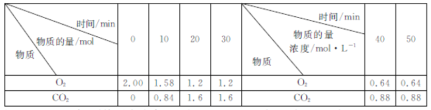
Θ®3Θ©άϊ”ΟCOΩ…“‘ΫΪNOΉΣΜ·ΈΣΈόΚΠΒΡN2Θ§ΤδΖ¥”ΠΈΣΘΚ2NOΘ®gΘ©ΘΪ2COΘ®gΘ©![]() N2Θ®gΘ©ΘΪ2CO2Θ®gΘ©Θ§œρ»ίΜΐΨυΈΣ1 LΒΡΦΉΓΔ““ΓΔ±ϊ»ΐΗωΚψΈ¬Θ®Ζ¥”ΠΈ¬Ε»Ζ÷±πΈΣ300 ΓφΓΔT ΓφΓΔ300 ΓφΘ©»ίΤς÷–Ζ÷±πΦ”»κœύΆ§ΝΩNOΚΆCOΘ§≤βΒΟΗς»ίΤς÷–nΘ®COΘ©ΥφΖ¥”Π ±ΦδtΒΡ±δΜ·«ιΩω»γœ¬±μΥυ ΨΘΚ
N2Θ®gΘ©ΘΪ2CO2Θ®gΘ©Θ§œρ»ίΜΐΨυΈΣ1 LΒΡΦΉΓΔ““ΓΔ±ϊ»ΐΗωΚψΈ¬Θ®Ζ¥”ΠΈ¬Ε»Ζ÷±πΈΣ300 ΓφΓΔT ΓφΓΔ300 ΓφΘ©»ίΤς÷–Ζ÷±πΦ”»κœύΆ§ΝΩNOΚΆCOΘ§≤βΒΟΗς»ίΤς÷–nΘ®COΘ©ΥφΖ¥”Π ±ΦδtΒΡ±δΜ·«ιΩω»γœ¬±μΥυ ΨΘΚ
t/min | 0 | 40 | 80 | 120 | 160 |
nΘ®COΘ©Θ®ΦΉ»ίΤςΘ©/mol | 2.0 | 1.5 | 1.1 | 0.8 | 0.8 |
nΘ®COΘ©Θ®““»ίΤςΘ©/mol | 2.0 | 1.45 | 1.0 | 1.0 | 1.0 |
nΘ®COΘ©Θ®±ϊ»ίΤςΘ©/mol | 1.00 | 0.80 | 0.65 | 0.53 | 0.45 |
ΔΌΦΉ»ίΤς÷–Θ§0ΓΪ40 minΡΎ”ΟNOΒΡ≈®Ε»±δΜ·±μ ΨΒΡΤΫΨυΖ¥”ΠΥΌ¬ vΘ®NOΘ©ΘΫ________ΓΘ
ΔΎΗΟΖ¥”ΠΒΡΠΛH________0Θ®ΧνΓΑ>Γ±ΜρΓΑ<Γ±Θ©ΓΘ
Δέ±ϊ»ίΤς¥οΒΫΤΫΚβ ±Θ§COΒΡΉΣΜ·¬ ΈΣ________ΓΘ
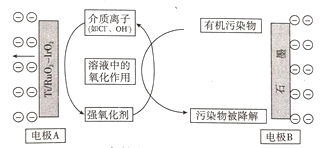
Θ®4Θ©»γΆΦ «‘ΎΥα–‘ΒγΫβ÷ »ή“Κ÷–Θ§“‘Εη–‘≤ΡΝœΉςΒγΦΪΘ§ΫΪCO2ΉΣΜ·ΈΣ±ϊœ©ΒΡ‘≠άμΡΘ–ΆΓΘ

ΔΌΧΪ―τΡήΒγ≥ΊΒΡΗΚΦΪ «________ΓΘΘ®ΧνΓΑaΓ±ΜρΓΑbΓ±Θ©
ΔΎ…ζ≥…±ϊœ©ΒΡΒγΦΪΖ¥”Π Ϋ «_________ΓΘ