جâؤ؟ؤعبف
،¾جâؤ؟،؟آب±½تاضطزھµؤسذ»ْ»¯¹¤²ْئ·£¬تاب¾ءد،¢ز½ز©،¢سذ»ْ؛د³ةµؤضذ¼نجه£¬¹¤زµةد³£سأ،°¼ند¶·¨،±ضئب،£¬·´س¦شہي،¢تµرé×°ضأح¼£¨¼سبب×°ضأ¶¼زرآشب¥£©بçدآ£؛![]()

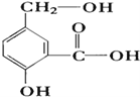
زرضھ£؛آب±½خھخقة«ز؛جه£¬·ذµم132.2،و£®
»ط´ًدآءذختجâ£؛
£¨1£©A·´س¦ئ÷تاہûسأتµرéتز·¨ضئب،آبئّ£¬ضذ؟ص²£ء§¹ـBµؤ×÷سأتا___________,ہنؤ¹ـضذہنث®س¦´س______£¨جî،°a،±»ٍ،°b،±£© ´¦ح¨بë،£
£¨2£©°ر¸ةشïµؤآبئّح¨بë×°سذ¸ةشï±½µؤ·´س¦ئ÷Cضذ£¨ؤعسذد൱سع±½ء؟1%µؤجْذ¼×÷´ك»¯¼ء£©£¬¼سببخ¬³ض·´س¦خآ¶بشع40،«60،وخھزث£¬خآ¶ب¹¸ك»لةْ³ة¶آب±½£®
¢ظ¶شC¼سببµؤ·½·¨تا_________£¨جîذٍ؛إ£©
a£®¾ئ¾«µئ¼سبب b£®سحش،¼سبب c£®ث®ش،¼سبب
¢عD³ِ؟عµؤئّجه³ة·ضسذHCl،¢________؛ح________£»
£¨3£©C·´س¦ئ÷·´س¦حê³ة؛َ£¬¹¤زµةدزھ½ّذذث®د´،¢¼îد´¼°ت³رخ¸ةش²إؤـصôءَ£®¼îد´ض®ا°زھث®د´µؤؤ؟µؤتاد´ب¥²؟·ضخق»ْخح¬ت±¼ُةظ¼îµؤسأء؟£¬½عش¼³ة±¾£®ذ´³ِسأ10%اâرُ»¯ؤئ¼îد´ت±؟ةؤـ·¢ةْµؤ»¯ر§·´س¦·½³جت½£؛_____________________£»_________________£»£¨ذ´ء½¸ِ¼´؟ة£©
£¨4£©ةدتِ×°ضأح¼ضذA،¢C·´س¦ئ÷ض®¼ن£¬ذèزھشِجيز»¸ِUذخ¹ـ£¬ئنؤعضأخïضتتا________________£»
£¨5£©¹¤زµةْ²ْضذ±½µؤء÷ت§اé؟ِبçدآ£؛
دîؤ؟ | ¶آب±½ | خ²ئّ | ²»ب·¶¨±½؛ؤ | ء÷ت§×ـء؟ |
±½ء÷ت§ء؟£¨kg/t£© | 13 | 24.9 | 51.3 | 89.2 |
شٍlt±½؟ةضئµأ³ةئ·خھ________________t£¨ض»زھاَءذت½£©،£
،¾´ً°¸،؟ئ½؛âئّر¹ a c ±½صôئّ آبئّ FeCl3+3NaOH=Fe£¨OH£©3،+3NaCl HCl+NaOH=NaCl+H2O خهرُ»¯¶ء×»ٍآب»¯¸ئ ![]()
،¾½âخِ،؟
£¨1£©²£ء§¹ـBةىبëز؛أودآ£¬×°ضأؤعر¹ا؟¹´َت±£¬؟ةزشسأسعئ½؛âئّر¹£»²ةب،ؤوء÷شہيح¨بëہنؤث®£¬ت¹ہنؤ¹ـ³نآْہنؤث®£¬³ن·ضہنب´£¬¼´ہنؤث®سةa؟عء÷ب룬سةb؟عء÷³ِ£¬¹ت´ً°¸خھئ½؛âئّر¹£»a£»
£¨2£©¢ظ·´س¦ئ÷C¼سبب؟طضئ·´س¦خآ¶بشع40-60،و£¬س¦ہûسأث®ش،¼سبب£¬¹تر،c£»
¢ع±½ز×»س·¢£¬·´س¦²ْةْµؤسذHCl£¬ازسذخ´·´س¦µؤآبئّ£¬D³ِ؟عئّجهضذ؛¬سذHCl،¢±½صôئّ،¢آبئّ£¬¹ت´ً°¸خھ±½صôئّ£»آبئّ£»
£¨3£©´ك»¯¼ءآب»¯جْسëاâرُ»¯ؤئ·´س¦£¬ةْ³ةµؤHCl»لسëاâرُ»¯ؤئ·´س¦£¬بـ½âµؤآبئّز²»لسëاâرُ»¯ؤئ·´س¦£¬·´س¦·½³جت½خھ£؛FeCl3+3NaOH=Fe£¨OH£©3،+3NaCl،¢HCl+NaOH=NaCl+H2O،¢Cl2+2NaOH=NaCl+NaClO+H2O£¬¹ت´ً°¸خھFeCl3+3NaOH=Fe£¨OH£©3،+3NaCl£»HCl+NaOH=NaCl+H2O£»
£¨4£©A،¢C·´س¦ئ÷ض®¼نذèزھشِجيUذح¹ـ£¬¸ةشïةْ³ةµؤآبئّ£¬؟ةزشسأخهرُ»¯¶ء×»ٍآب»¯¸ئµب£¬¹ت´ً°¸خھخهرُ»¯¶ء×»ٍآب»¯¸ئ£»
£¨5£©±½µؤ×ـء÷ت§ء؟خھ89.2kg/t£¬¹ت1t±½ضذ²خ¼س·´س¦µؤ±½µؤضتء؟خھ£¨1t-0.0892t£©£¬سة±½سëآب±½ضتء؟ض®±بخھ78:112.5؟ةضھةْ³ةآب±½ضتء؟خھ![]() t،£
t،£

،¾جâؤ؟،؟ز»ضضزشبيأج؟َ(ض÷زھ³ة·ضخھMnO2،¢SiO2،¢Al2O3)؛ح»ئجْ؟َ(FeS2،¢SiO2)خھشءدز±ء¶½ًتôأجµؤ¹¤زصء÷³جبçح¼ثùت¾£؛

زرضھدà¹ط½ًتôہë×س[c(Mn£«)£½0.1mol،¤L£1]ذخ³ةاâرُ»¯خï³ءµيµؤpHبçدآ£؛
½ًتôہë×س | Fe3£« | Mn2£« | Al3£« |
؟ھت¼³ءµيµؤpH | 2.7 | 8.3 | 3.2 |
³ءµيحêب«µؤpH | 3.7 | 9.8 | 5.0 |
»ط´ًدآءذختجâ£؛
³شس²½ضèضذ¼سبëµؤMnCO3µؤ×÷سأتا____________________________£¬¸أ¹³جذèµ÷½عبـز؛pHµؤ·¶خ§تا___________________________£¬بô°رpHµ÷µأ¹¸ك£¬ئن؛َ¹ûتا_________________________،£