��Ŀ����
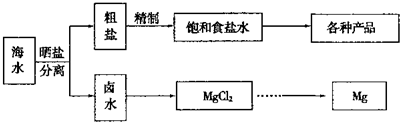
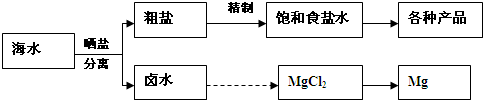
��ˮ���̲��ŷḻ����Դ����ˮ�ۺ����õ�����ͼ���¡�
(1)��NaCl��ԭ�Ͽ��Եõ����ֲ�Ʒ��
�ٹ�ҵ����NaCl�Ʊ������ƵĻ�ѧ����ʽ��_____________________________________��
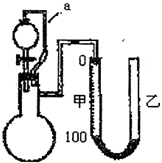
��ʵ�����ö��Ե缫���100 mL 0.1 mol��L-1 NaCl��Һ�����������������õ�112 mL����(��״��)����������Һ��pHΪ_________(���Է�Ӧǰ����Һ������仯)��
�۵���Ȼ���ϡ��Һ���Ʊ���84����Һ����ͨ��ʱ��������Һ��ȫ���գ�����������Һ����һ�����ʣ�д����Ӧ�Ļ�ѧ����ʽ��______________________________________________��
(2)��������κ��±ˮ���̺��ŷḻ��þ��Դ����ת����ɻ��MgCl2�ֲ�Ʒ��
���� | ��ʼ���� | ������� |
Fe(OH)3 | 2.7 | 3.7 |
Fe(OH)2 | 7.6 | 9.6 |
Mn(OH)2 | 8.3 | 9.8 |
Mg(OH)2 | 9.6 | 11.1 |
�ٴֲ�Ʒ����Һ�к���Na+��Fe2+��Fe3+��Mn2+���轫Fe2+��Fe3+��Mn2+ת��Ϊ������ȥ��Fe(OH)2����״��������ת��ΪFe(OH)3����ȥ(�����������������pH���ϱ�)����ֻ��һ������(1)�еõ��IJ�Ʒ�������ʵĻ�ѧʽΪ_____________��������Һ��pHΪ____________________��
���ڼ��õ���ˮ��������ڵ�������Ƕþ�������õ绯ѧԭ������ֹ�ڵ���ʴ��д�������ĵ缫��Ӧʽ��______________________________��
(1)��2NaCl![]() 2Na+Cl2�� ��13
2Na+Cl2�� ��13
��NaCl+H2O![]() NaClO+H2��
NaClO+H2��
(2)��NaClO 9.8
��O2+2H2O+4e-![]() 4OH-
4OH-
������(1)��
2NaCl+2H2O![]() 2NaOH+H2��+Cl2��
2NaOH+H2��+Cl2��
2 mol
n(OH-)
n(OH-)=![]() =0.01 mol��NaClǡ����ȫ��⣬��Һ��pH=13.
=0.01 mol��NaClǡ����ȫ��⣬��Һ��pH=13.
��2NaCl+2H2O![]() 2NaOH+H2+Cl2
2NaOH+H2+Cl2
Cl2+2NaOH![]() NaCl+NaClO+H2O
NaCl+NaClO+H2O
������������ʽ��ӿɵ�NaCl+H2O![]() NaClO+H2��.
NaClO+H2��.
(2)��������ʼ�Ҫ�������ԣ����ܵ�����ҺpH��ֻ��ѡ��NaClO��NaClO�ڽ�Fe2+������ͬʱ����OH-��ʹFe3+ת��Ϊ������ҪʹFe3+��Mn2+��ȫ������Ӧ������Һ��pH=9.8.