��Ŀ����
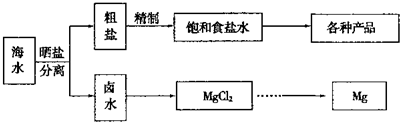
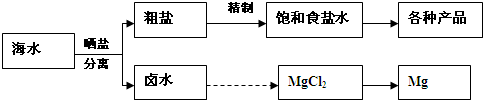
��ˮ���̲��ŷḻ����Դ��������Ҫ�ĺܶ���϶���Դ�ں�ˮ���ۺ����ã�
��1�������йغ�ˮ�ۺ����õ�˵������ȷ����
A��ֻ��ͨ�������仯���ɴӺ�ˮ�еõ��ص���
B����ˮͨ�������ɵõ���ˮ��ͨ������ɵõ�����
C���Ӻ�ˮ�п���ȡ�弰�仯����Ʒ
D�����ó�ϫ�����ǽ���ѧ��ת��Ϊ����
��2��д����ҵ����MgCl2�Ʊ�����þ�Ļ�ѧ����ʽ
��3����ˮ�зḻ���Ȼ�������Ҫ�Ļ���ԭ�ϣ����ⱥ��ʳ��ˮ���Ʊ����ֻ�����Ʒ��
����ʯī�缫��ⱥ��ʳ��ˮʱ�������ĵ缫��Ӧʽ��
�����ö��Ե缫��ⱥ��ʳ��ˮ�����Ʊ���84������Һ����Ҫ�ɷ���NaClO����ͨ��ʱ�������������屻��Һ��ȫ���գ�����������Һ����һ�����ʣ������ܻ�ѧ����ʽ��
��4����ҵ������һ����װ�õ��K2SO4��Һ����ȡH2��O2��H2SO4��KOH��������Щ�����������ȼ�ϵ�أ����ظ����ĵ缫��Ӧ����ʽΪ
��1�������йغ�ˮ�ۺ����õ�˵������ȷ����
C
C
��A��ֻ��ͨ�������仯���ɴӺ�ˮ�еõ��ص���
B����ˮͨ�������ɵõ���ˮ��ͨ������ɵõ�����
C���Ӻ�ˮ�п���ȡ�弰�仯����Ʒ
D�����ó�ϫ�����ǽ���ѧ��ת��Ϊ����
��2��д����ҵ����MgCl2�Ʊ�����þ�Ļ�ѧ����ʽ
MgCl2
Mg+Cl2��
| ||
MgCl2
Mg+Cl2��
��
| ||
��3����ˮ�зḻ���Ȼ�������Ҫ�Ļ���ԭ�ϣ����ⱥ��ʳ��ˮ���Ʊ����ֻ�����Ʒ��
����ʯī�缫��ⱥ��ʳ��ˮʱ�������ĵ缫��Ӧʽ��
2Cl--2e-=Cl2��
2Cl--2e-=Cl2��
�������ö��Ե缫��ⱥ��ʳ��ˮ�����Ʊ���84������Һ����Ҫ�ɷ���NaClO����ͨ��ʱ�������������屻��Һ��ȫ���գ�����������Һ����һ�����ʣ������ܻ�ѧ����ʽ��
NaCl+H2O
NaClO+H2��
| ||
NaCl+H2O
NaClO+H2��
����84������Һ�ڳ����µ�pH
| ||
��
��
7�������ӷ���ʽ��ʾ��ԭ��ClO-+H2O?HClO+OH-
ClO-+H2O?HClO+OH-
����4����ҵ������һ����װ�õ��K2SO4��Һ����ȡH2��O2��H2SO4��KOH��������Щ�����������ȼ�ϵ�أ����ظ����ĵ缫��Ӧ����ʽΪ
H2-2e-+2OH-=2H2O��H2-2e-=2H+
H2-2e-+2OH-=2H2O��H2-2e-=2H+
����������1��A��KԪ���ں�ˮ����Ҫ�Լ�������ʽ���ڣ�
B����ˮͨ�������ɵõ����Σ�ͨ������ɵõ���ˮ��
C����ˮ�к����廯��Ӻ�ˮ�п���ȡ�弰�仯����Ʒ��
D�����ó�ϫ���磬û�з�����ѧ��Ӧ��
��2����ҵ���õ�����ڵ�MgCl2�Ʊ�����þ��������ڵ��Ȼ�þ����þ��������
��3������������������Ӧ���������������ŵ�����������
���������֪����ⱥ��ʳ��ˮ�Ʊ���84������Һ�����ɴ���������������
����������ǿ�������Σ��������ˮ�⣬��Һ�ʼ��ԣ�
��4����������������Ӧ�������ڸ����ŵ磬�ڼ�������������ˮ���������������������ӣ�
B����ˮͨ�������ɵõ����Σ�ͨ������ɵõ���ˮ��
C����ˮ�к����廯��Ӻ�ˮ�п���ȡ�弰�仯����Ʒ��
D�����ó�ϫ���磬û�з�����ѧ��Ӧ��
��2����ҵ���õ�����ڵ�MgCl2�Ʊ�����þ��������ڵ��Ȼ�þ����þ��������
��3������������������Ӧ���������������ŵ�����������
���������֪����ⱥ��ʳ��ˮ�Ʊ���84������Һ�����ɴ���������������
����������ǿ�������Σ��������ˮ�⣬��Һ�ʼ��ԣ�
��4����������������Ӧ�������ڸ����ŵ磬�ڼ�������������ˮ���������������������ӣ�
����⣺��1��A��KԪ���ں�ˮ����Ҫ�Լ�������ʽ���ڣ�����ͨ����ѧ�仯���K���ʣ���A����
B����ˮͨ�������ɵõ����Σ�ͨ������ɵõ���ˮ����B����
C����ˮ�к����廯��Ӻ�ˮ�п���ȡ�弰�仯����Ʒ����C��ȷ��
D�����ó�ϫ�����dz�ϫ��ת��Ϊ���ܣ�û�з�����ѧ��Ӧ������ѧ��ת��Ϊ���ܱ���Ҫ������ѧ��Ӧ����D����
�ʴ�Ϊ��C��
��2����ҵ���õ�����ڵ�MgCl2�Ʊ�����þ��������ڵ��Ȼ�þ����þ����������Ӧ��ѧ����ʽΪMgCl2
Mg+Cl2����
�ʴ�Ϊ��MgCl2
Mg+Cl2����
��3������������������Ӧ���������������ŵ������������缫��ӦʽΪ��2Cl--2e-=Cl2����
�ʴ�Ϊ��2Cl--2e-=Cl2����
���������֪����ⱥ��ʳ��ˮ�Ʊ���84������Һ�����������������������ɵ��������������Ʒ�Ӧ���ɴ������ơ��Ȼ��ƣ������������ɴ�����������������Ӧ���ܷ���ʽΪ��NaCl+H2O
NaClO+H2����
����������ǿ�������Σ��������ˮ��ClO-+H2O?HClO+OH-���ƻ�ˮ�ĵ���ƽ�⣬��Һ�ʼ��ԣ���pH��7��
�ʴ�Ϊ��NaCl+H2O
NaClO+H2��������ClO-+H2O?HClO+OH-��
��4����������������Ӧ�������ڸ����ŵ磬�ڼ�������������ˮ���缫��ӦʽΪ��H2-2e-+2OH-=2H2O��
�������������������ӣ��缫��ӦʽΪ��H2-2e-=2H+��
�ʴ�Ϊ��H2-2e-+2OH-=2H2O��H2-2e-=2H+��
B����ˮͨ�������ɵõ����Σ�ͨ������ɵõ���ˮ����B����
C����ˮ�к����廯��Ӻ�ˮ�п���ȡ�弰�仯����Ʒ����C��ȷ��
D�����ó�ϫ�����dz�ϫ��ת��Ϊ���ܣ�û�з�����ѧ��Ӧ������ѧ��ת��Ϊ���ܱ���Ҫ������ѧ��Ӧ����D����
�ʴ�Ϊ��C��
��2����ҵ���õ�����ڵ�MgCl2�Ʊ�����þ��������ڵ��Ȼ�þ����þ����������Ӧ��ѧ����ʽΪMgCl2
| ||
�ʴ�Ϊ��MgCl2
| ||
��3������������������Ӧ���������������ŵ������������缫��ӦʽΪ��2Cl--2e-=Cl2����
�ʴ�Ϊ��2Cl--2e-=Cl2����
���������֪����ⱥ��ʳ��ˮ�Ʊ���84������Һ�����������������������ɵ��������������Ʒ�Ӧ���ɴ������ơ��Ȼ��ƣ������������ɴ�����������������Ӧ���ܷ���ʽΪ��NaCl+H2O
| ||
����������ǿ�������Σ��������ˮ��ClO-+H2O?HClO+OH-���ƻ�ˮ�ĵ���ƽ�⣬��Һ�ʼ��ԣ���pH��7��
�ʴ�Ϊ��NaCl+H2O
| ||
��4����������������Ӧ�������ڸ����ŵ磬�ڼ�������������ˮ���缫��ӦʽΪ��H2-2e-+2OH-=2H2O��
�������������������ӣ��缫��ӦʽΪ��H2-2e-=2H+��
�ʴ�Ϊ��H2-2e-+2OH-=2H2O��H2-2e-=2H+��
���������⿼�麣ˮ��Դ���ۺ����á����ԭ����ȼ�ϵ�ء����û�ѧ����ȣ��Ѷ��еȣ�ע�����������ԭ��Ӧ����ԭ��������ԭ����

��ϰ��ϵ�д�
�����Ŀ