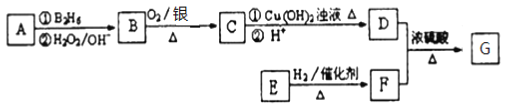
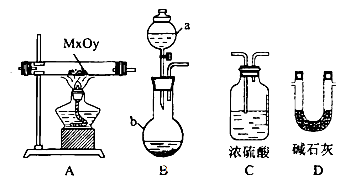
��Ŀ����
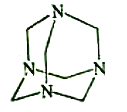
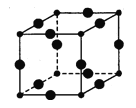
����Ŀ���������ڵ�����������γ�������ѧ��ͨ��X���߲�õ����ṹʾ��ͼ�ɼ�ʾ���£�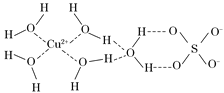
��1��Cu��̬ԭ�ӵ���Χ�����Ų�Ϊ ��Cr��̬ԭ�ӵ���Χ�����Ų�Ϊ �������Ų���������_____________________��
��2��NH3��һ�ֺܺõ����壬ԭ����______________________��
��3��ͼ�����߱�ʾ��������Ϊ________________��
��4��������Һ�백ˮ��һ�������¿�������Cu(NH3)4SO4��H2O���塣�ڸþ����У����е�ԭ���Ż�����У�[Cu(NH3)4]2����NH3��SO42����H2O������[Cu(NH3)4]2��Ϊƽ�������νṹ�������������ṹ��ԭ���Ż������__________��д��һ�������������ṹ��Ϊ�ȵ�����ķ��ӵķ���ʽ ��
��5������������CO�����������ȣ�������ɫ�ӷ���Һ̬Ni(CO)4�����������幹�͡�Ni(CO)4������________(����)��
A��ˮ | B�����Ȼ�̼ | C���� | D����������Һ |
���𰸡���1��3d104s13d54s13d���ȫ������������ͽ��ȶ�
��2����ԭ����һ�Թµ��Ӷԣ�3���������λ����4��SO42��CCl4��5��BC
�������������������1��ͭ��ԭ��������29����̬ԭ�ӵ���Χ�����Ų�Ϊ3d104s1��Cr��ԭ������Ϊ24����̬ԭ�ӵ���Χ�����Ų�Ϊ3d54s1�����ݺ��ع����֪3d���ȫ������������ͽ��ȶ�����2�����ڵ�ԭ����һ�Թµ��Ӷԣ�����NH3��һ�ֺܺõ����壻��3��Cu2+��O֮��Ϊ��λ����H��OΪ�������4��S��4��O�γɹ��ۼ�������4���������µ��Ӷ���=![]() =0����˿ռ乹��Ϊ��������ṹ��ԭ�����ͼ۵������ֱ���ȵ��ǵȵ����壬�����������Ϊ�ȵ��������CCl4����5������������CO�����������ȣ�������ɫ�ӷ���Һ̬Ni(CO)4��ӦΪ���Ӿ��壬���������幹�ͣ�ӦΪ�Ǽ��Է��ӣ������ڷǼ����ܼ���ˮ�Ǽ��Է��ӣ����Ȼ�̼�ͱ��ǷǼ��Է��ӣ���ѡBC��
=0����˿ռ乹��Ϊ��������ṹ��ԭ�����ͼ۵������ֱ���ȵ��ǵȵ����壬�����������Ϊ�ȵ��������CCl4����5������������CO�����������ȣ�������ɫ�ӷ���Һ̬Ni(CO)4��ӦΪ���Ӿ��壬���������幹�ͣ�ӦΪ�Ǽ��Է��ӣ������ڷǼ����ܼ���ˮ�Ǽ��Է��ӣ����Ȼ�̼�ͱ��ǷǼ��Է��ӣ���ѡBC��

 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�