��Ŀ����
����Ŀ��Ϊ�˱�����������ѧ���������о�������Ⱦ���������
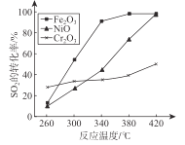
��1��������CO��SO2���̵�����Fe2O3����������CO��SO2��380��ʱת��ΪS��һ�������塣��֪��������۵���112.8�����е���444.6�����ڷ�Ӧÿ�õ�mol�����ų� 270kJ��������д���������̵�����Ӧ���Ȼ�ѧ����ʽ____________________________________������������ͬ��������ͬʱ��������Ӧ��SO2��ת�����淴Ӧ�¶ȵı仯��ͼ��ʾ�������Ǵ����۸����أ�������ѡFe2O3����������Ҫԭ���� ____________________________________��
��2����450��������V2O5�Ĵ���������SO2��ת��ΪSO3��2SO2(g)+O2 (g)![]() 2SO3(g) ��H= -190kJ��mol -1����֪�������ȼ����Ϊ296kJ��mol -1���� S(s)+
2SO3(g) ��H= -190kJ��mol -1����֪�������ȼ����Ϊ296kJ��mol -1���� S(s)+![]() O2(g)
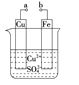
O2(g)![]() SO3(g) ��H=___kJ��mol -1 ����ͭ����������ɹ�ҵβ����SO2�IJ��ִ���������������ӦΪ��2SO2+2nCu+(n+1)O2+(2-2n)H2O =2nCuSO4 +(2-2n)H2SO4 �ӻ��������ĽǶȿ�,�����������Ϊ__________��ÿ���ձ�״����11.2 LSO2����SO2��ԭ��O2������Ϊ__________g��
SO3(g) ��H=___kJ��mol -1 ����ͭ����������ɹ�ҵβ����SO2�IJ��ִ���������������ӦΪ��2SO2+2nCu+(n+1)O2+(2-2n)H2O =2nCuSO4 +(2-2n)H2SO4 �ӻ��������ĽǶȿ�,�����������Ϊ__________��ÿ���ձ�״����11.2 LSO2����SO2��ԭ��O2������Ϊ__________g��
��3����һ���̶��ݻ�Ϊ5L���ܱ������г�0.20molSO2��0.10molO2����450�����д��������£�����Ӻ�ﵽƽ�⣬��������к�SO30.10 mol����V(O2)=______(mol��L -1��min -1 )���ų�������Ϊ______kJ�����¶��µ�ƽ�ⳣ��Ϊ________��������ͨ��0.20 molSO2��0.10mol O2�����ٴ�ƽ��ʱ�����������ת����________(ѡ������������С�����䡱)��
���𰸡���1��2CO(g)+SO2(g) =S(l)+2CO2(g) ��H = -270kJ��mol -1 Fe2O3������������Խϵ��¶��£���λʱ���ڻ�ýϸߵ�SO2ת���ʣ��ܺ�С ��2��-391 ��ֹ����ķ��� 8.0
��3��0.02 9.5 100 ���
��������
�����������1���ȸ�������д����Ӧ�ķ���ʽ(����ע�����ʵ�״̬)��2CO(g)+SO2(g) =S(l)+2CO2(g)������ÿ�õ�1mol��Ӧ�ų�270kJ ���������÷�Ӧ����H = -270kJ��mol -1���ݴ˿�д�����Ȼ�ѧ����ʽ����ͼ��֪��Fe2O3������������Խϵ��¶��£���λʱ���ڻ�ýϸߵ�SO2ת���ʣ��ܺ�С���������ܼ��ٶ���������ŷţ�������������Ŀ����ԡ���2���������������Ȼ�ѧ����ʽ�����ø�˹���ɡ�����1ʽ��1/2+2ʽ��������Ӧ���Ȼ�ѧ�����е���H=-391kJ��mol-1�����ݹ�ϵʽ��2SO2~4e-~O2���ɼ��������������������3�����ݶ���������������Ӧ��ϵ������������ɼ����V(O2)=![]() = 0.02mol��L -1��min -1����2SO2 (g)+O2(g )
= 0.02mol��L -1��min -1����2SO2 (g)+O2(g )![]() 2SO3(g) ��H=-190kJ��mol-1������0.1 molSO3����ʱ���ų�����190��1/2=9.5kJ�������ʵ�ƽ��Ũ��c(SO2)= 0.02mol��L -1��c(O 2 )=0.01mol��L -1��c (SO3) = 0.02mol��L-1��������ƽ�ⳣ��K =
2SO3(g) ��H=-190kJ��mol-1������0.1 molSO3����ʱ���ų�����190��1/2=9.5kJ�������ʵ�ƽ��Ũ��c(SO2)= 0.02mol��L -1��c(O 2 )=0.01mol��L -1��c (SO3) = 0.02mol��L-1��������ƽ�ⳣ��K =![]() =100�� ������������������Ӧ���ʵ����ʵ����������ӱ�����������൱�ڼ�ѹ��ƽ��������Ӧ�������,���������ת���ʱ��
=100�� ������������������Ӧ���ʵ����ʵ����������ӱ�����������൱�ڼ�ѹ��ƽ��������Ӧ�������,���������ת���ʱ��