��Ŀ����
2��X��Y���Ƿ����廯�����Ϊ����ʳ���㾫���㷺���ڻ�ױƷ���ǹ�����ζƷ�У�1mol Xˮ��õ�1mol Y��1mol CH3CH2OH��X��Y�ķ�������������200����ȫȼ�ն�ֻ����CO2��H2O����X������̼����Ԫ���ܵ������ٷֺ���ԼΪ81.8%����1��X�ķ���ʽ��C11H12O2��
��2��X��Y������֮��Ϊ28��
��3��1��Y������Ӧ����2����ԭ�ӣ�
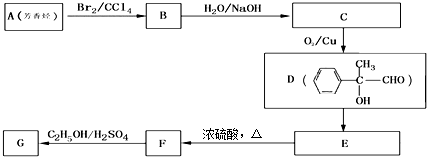
��4��G��X��Ϊͬ���칹�壬�Ҿ�����ͬ�Ĺ����ţ��÷�����A�ϳ�G·����ͼ��
��д��A�Ľṹ��ʽ
��E��F�ķ�Ӧ��������ȥ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ��
 ����д�����з�������������F��ͬ���칹��Ľṹ��ʽ��
����д�����з�������������F��ͬ���칹��Ľṹ��ʽ��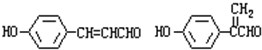 ��
�����������ڳ��˱�����������״�ṹ���ұ�������2����λȡ������
����һ�������£������ʼ�����������Һ����������Ӧ���ܺ�FeCl3��Һ������ɫ��Ӧ��
���� 1molXˮ��õ�1molY��1mol CH3CH2OH��X��Y����Է���������������200����ȫȼ�ն�ֻ����CO2��H2O����X������̼����Ԫ���ܵ������ٷֺ���ԼΪ81.8%����OΪ
$\frac{200����1-81.8%��}{16}$��2����XΪ������D�Ľṹ��֪��D��E������������EΪ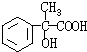 ��E��F������ȥ��Ӧ������FΪ
��E��F������ȥ��Ӧ������FΪ ��F���Ҵ�����������Ӧ����G����GΪ
��F���Ҵ�����������Ӧ����G����GΪ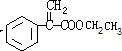 ��G��X��Ϊͬ���칹�壬�Ҿ�����ͬ�Ĺ����ţ���XΪ
��G��X��Ϊͬ���칹�壬�Ҿ�����ͬ�Ĺ����ţ���XΪ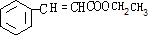 ��YΪ
��YΪ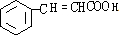 ���ٽ�Ϻϳ�·�߿�֪��A��B�����ӳɣ�B��C����ˮ�⣬C��D��������������AΪ
���ٽ�Ϻϳ�·�߿�֪��A��B�����ӳɣ�B��C����ˮ�⣬C��D��������������AΪ ���ݴ˷������
���ݴ˷������
��� �⣺1molXˮ��õ�1molY��1mol CH3CH2OH��X��Y����Է���������������200����ȫȼ�ն�ֻ����CO2��H2O����X������̼����Ԫ���ܵ������ٷֺ���ԼΪ81.8%����OΪ$\frac{200����1-81.8%��}{16}$��2����XΪ������D�Ľṹ��֪��D��E������������EΪ ��E��F������ȥ��Ӧ������FΪ
��E��F������ȥ��Ӧ������FΪ ��F���Ҵ�����������Ӧ����G����GΪ
��F���Ҵ�����������Ӧ����G����GΪ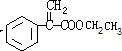 ��G��X��Ϊͬ���칹�壬�Ҿ�����ͬ�Ĺ����ţ���XΪ
��G��X��Ϊͬ���칹�壬�Ҿ�����ͬ�Ĺ����ţ���XΪ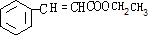 ��YΪ
��YΪ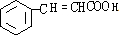 ���ٽ�Ϻϳ�·�߿�֪��A��B�����ӳɣ�B��C����ˮ�⣬C��D��������������AΪ
���ٽ�Ϻϳ�·�߿�֪��A��B�����ӳɣ�B��C����ˮ�⣬C��D��������������AΪ ��
��
��1��XΪ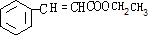 �������ʽΪC11H12O2���ʴ�Ϊ��C11H12O2��
�������ʽΪC11H12O2���ʴ�Ϊ��C11H12O2��
��2��XΪ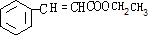 ��YΪ
��YΪ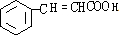 ������X��Y������֮��Ϊ28��
������X��Y������֮��Ϊ28��
�ʴ�Ϊ��28��
��3��YΪ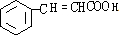 ��Y�к�������Oԭ�ӣ�
��Y�к�������Oԭ�ӣ�
�ʴ�Ϊ��2��
��4����ͨ�����Ϸ���֪��A�Ľṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��E��Ũ���������������������·�����ȥ��Ӧ����F����Ӧ����ʽΪ ��
��
�ʴ�Ϊ����ȥ�� ��
��
��FΪ ��F��ͬ���칹���������������
��F��ͬ���칹���������������
i�������ڳ��˱��������������ṹ���ұ�������2����λȡ������˵����������ȡ������λ�ڶ�λ��
ii��һ�������£������ʼ�����������Һ����������ӦҲ�ܺ�FeCl3��Һ������ɫ��Ӧ˵������ȩ���ͷ��ǻ��������������Fͬ���칹��Ϊ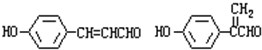 ��
��
�ʴ�Ϊ��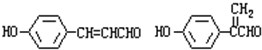 ��
��
���� ���⿼���л����ƶϣ����ؿ�������ƶ���������DΪͻ�ƿڲ��������ϵķ��������ƶϣ��������ճ����л�������ż�������֮��Ĺ�ϵ���ѵ���������ͬ���칹��������жϼ��ṹ��ʽ����д����Ŀ�Ѷ��еȣ�

| A�� | H2O | B�� | HCl | C�� | MgCl2 | D�� | SO2 |
| A�� | ������ȼ����Ϊ285.8 kJ/mol����ˮ�����Ȼ�ѧ����ʽΪ��2H2O��l��=2H2��g��+O2��g����H=+285.8 kJ/mol | |
| B�� | 1 mol������ȫȼ������CO2��H2O��l��ʱ�ų�890kJ������������ȼ���Ȼ�ѧ����ʽΪ��$\frac{1}{2}$CH4��g��+O2��g��=$\frac{1}{2}$CO2 ��g��+H2O��l����H=-445 kJ/mol | |
| C�� | HF��NaOH��Һ��Ӧ��H+��aq��+OH-��aq��=H2O��l����H=-57.3 kJ/mol | |
| D�� | ��ʯī�Ƚ��ʯ�ȶ���֪��C�����ʯ��s��=C��ʯī��s����H��0 |
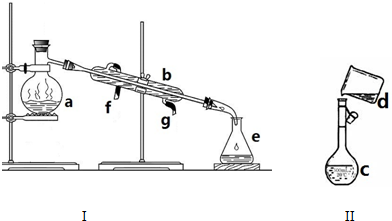
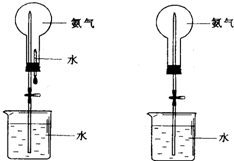 ��ȡ�����������Ȫʵ�飨ͼ�мг�װ�þ�����ȥ����
��ȡ�����������Ȫʵ�飨ͼ�мг�װ�þ�����ȥ���� ��1���Ӳ�ͬ�ĽǶȣ���ѧ��Ӧ�����в�ͬ�ķ��������ͬ���Ļ�ѧ��Ӧ������һ������ϵ��������ͼ�ķ����У���ͼʾ��ÿ��ԲȦ����һ��
��1���Ӳ�ͬ�ĽǶȣ���ѧ��Ӧ�����в�ͬ�ķ��������ͬ���Ļ�ѧ��Ӧ������һ������ϵ��������ͼ�ķ����У���ͼʾ��ÿ��ԲȦ����һ��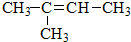
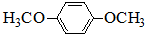
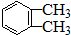
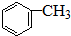
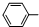 ��
��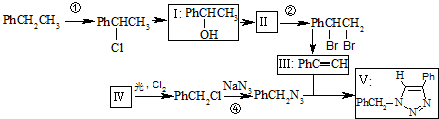
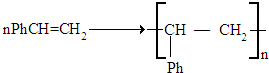 ����Ҫ��д����Ӧ��������
����Ҫ��д����Ӧ��������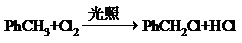 ��Ҫ��д����Ӧ��������
��Ҫ��д����Ӧ��������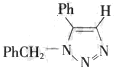 ��
��