��Ŀ����
4���߷��Ӳ���PET������֬��PMMA�ĺϳ�·�����£�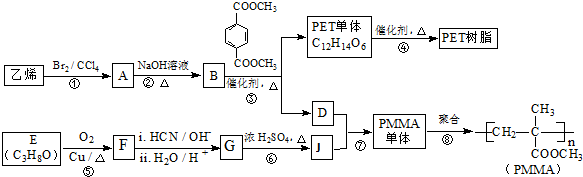 ��֪��
��֪����RCOOR��+R��18OH$?_{��}^{����}$RCO18OR��+R��OH��R��R�䡢R�����������
��
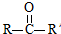 $��_{ii��H_{2}O/H+}^{i��HCN/OH-}$
$��_{ii��H_{2}O/H+}^{i��HCN/OH-}$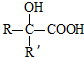 ��R��R������������
��R��R��������������1���ٵķ�Ӧ�����Ǽӳɷ�Ӧ��
��2���ڵĻ�ѧ����ʽΪ
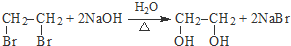 ��
����3��F�ĺ˴Ź���������ʾֻ��һ��壬�ݵĻ�ѧ����ʽΪ
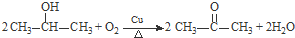 ��
����4��G�Ľṹ��ʽΪ
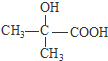 ��
����5������˵����ȷ����cd������ĸ��ţ���
a��1mol
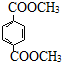 ������NaOH��Һ��Ӧʱ���������4mol NaOH
������NaOH��Һ��Ӧʱ���������4mol NaOHb��B��D��Ϊͬϵ�� ������c����Ϊ������Ӧ
d��D�ķе��̼ͬԭ������������
��6����J������ͬ�����ŵ�J��ͬ���칹���ж��֣���д����������һ��CH2=CHCH2COOH��
���� �������и�����ת����ϵ����PMMA�Ľṹ��֪��PMMA����ΪCH2=C��CH3��COOCH3��E������F��F�ĺ˴Ź���������ʾֻ��һ��壬F������Ϣ���еķ�Ӧ��G��G��Ũ���������·�����ȥ��Ӧ��J�����PMMA����Ľṹ��E�ķ���ʽ��֪��EΪCH3CHOHCH3��FΪCH3COCH3��GΪ��CH3��2COHCOOH��JΪCH2=C��CH3��COOH������DΪHOCH3����ϩ���巢���ӳɷ�Ӧ����AΪBrCH2CH2Br��A�ڼ���������ˮ���BΪHOCH2CH2OH��B��Ա��������������ȡ����Ӧ����PET����Ϊ ��PET���巢����Ϣ��ķ�Ӧ��PET�������ݴ˴��⣮
��PET���巢����Ϣ��ķ�Ӧ��PET�������ݴ˴��⣮
��� �⣺�������и�����ת����ϵ����PMMA�Ľṹ��֪��PMMA����ΪCH2=C��CH3��COOCH3��E������F��F�ĺ˴Ź���������ʾֻ��һ��壬F������Ϣ���еķ�Ӧ��G��G��Ũ���������·�����ȥ��Ӧ��J�����PMMA����Ľṹ��E�ķ���ʽ��֪��EΪCH3CHOHCH3��FΪCH3COCH3��GΪ��CH3��2COHCOOH��JΪCH2=C��CH3��COOH������DΪHOCH3����ϩ���巢���ӳɷ�Ӧ����AΪBrCH2CH2Br��A�ڼ���������ˮ���BΪHOCH2CH2OH��B��Ա��������������ȡ����Ӧ����PET����Ϊ ��PET���巢����Ϣ��ķ�Ӧ��PET������
��PET���巢����Ϣ��ķ�Ӧ��PET������
��1����������ķ�����֪����Ӧ�ٵķ�Ӧ�����Ǽӳɷ�Ӧ��
�ʴ�Ϊ���ӳɷ�Ӧ��
��2���ڵĻ�ѧ����ʽΪ 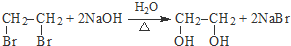 ��
��
�ʴ�Ϊ��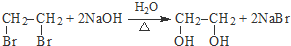 ��
��
��3����Ӧ�ݵĻ�ѧ����ʽΪ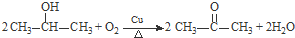 ��
��
�ʴ�Ϊ��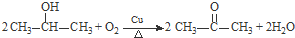 ��
��
��4����������ķ�����֪��G�Ľṹ��ʽΪ  ��
��
�ʴ�Ϊ�� ��
��
��5��a.1mol  ������NaOH��Һ��Ӧʱ���������2mol NaOH����a����
������NaOH��Һ��Ӧʱ���������2mol NaOH����a����
b��DΪHOCH3��BΪHOCH2CH2OH�����ǵ��ǻ�����Ŀ��ͬ������B��D���ǻ�Ϊͬϵ���b����
c����ΪCH2=C��CH3��COOH��HOCH3����������Ӧ����c��ȷ��
d��D�����ǻ������γ����������D�ķе��̼ͬԭ�����������ߣ���d��ȷ��
��ѡcd��
��6��J��ij��ͬ���칹����J������ͬ�����ţ���������һ�ֽṹ��ʽ��CH2=CHCH2COOH��
�ʴ�Ϊ��CH2=CHCH2COOH��
���� ���⿼���л�����ƶ���ϳɣ�ע����ݳ������������Ϣ���л���Ľṹ�����ƶϣ���ȷ�л���Ĺ����ż��������ǽⱾ��ؼ����Ѷ��еȣ�

| A�� | ���������ܶȺ㶨���� | B�� | ����������ɫ���ٸı� | ||
| C�� | H2��I2��HI��Ũ����� | D�� | ���������ʵ������� |
| A�� | NH4Cl��s��=NH3��g��+HCl��g�������²����Է����У�˵���÷�Ӧ�ġ�H��0 | |
| B�� | ��������Ʒ�Ʋ����������Ʒ������ǰ���������⣬����п����Ʒ���෴ | |
| C�� | ����ˮ�������ϸ��¶ȣ�K���pH��С�������� | |
| D�� | ��0.1mol•L-1CH3COOH��Һ�м��ȣ���c��H+����c��CH3COOH���ı�ֵ���� |
| A�� | ��ϩ����ϩ | B�� | �״����Ҷ��� | C�� | ��Ȳ���� | D�� | ��ϩ�������� |
| A�� | NaOH | B�� | AlCl3 | C�� | K2S | D�� | Cl2 |
| A�� | ��NaCl���һ�����ʵ���Ũ����Һ | B�� | HCl����ˮ | ||
| C�� | ˮ���ˮ���� | D�� | ��NH4��2CO3��ǿ�� |
| A�� | Na2S | B�� | CaCl2 | C�� | Na2O2 | D�� | H2O2 |
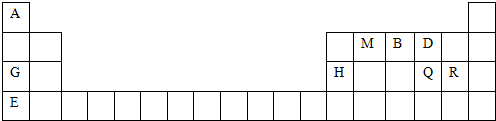
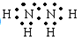 ����������Ľṹʽ��
����������Ľṹʽ�� ��
��
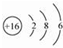
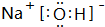 �� �õ���ʽ��ʾG��Q�γɻ�����Ĺ���
�� �õ���ʽ��ʾG��Q�γɻ�����Ĺ���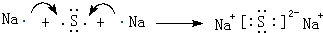 ��
��