��Ŀ����
����Ŀ���⡢������Ԫ����ɵij���������H2O��H2O2��������һ�������¾��ɷֽ⡣
��1����֪��
��ѧ�� | �Ͽ�1mol��ѧ�������������kJ�� |
H-H | 436 |
O-H | 463 |
O=O | 498 |
��H2O�ĵ���ʽ��________________��
��H2O(g)�ֽ���Ȼ�ѧ����ʽ��________________________��
��11.2 L����״������H2��ȫȼ�գ�������̬ˮ���ų�__________kJ��������
��2��ijͬѧ��H2O2�ֽ�Ϊ����̽��Ũ������Һ����ԶԷ�Ӧ���ʵ�Ӱ���������£����������ʾ�ķ������ʵ����
ʵ���� | ��Ӧ�� | ���� | |
a | 50 mL5%H2O2��Һ | 1 mL0.1 mol��L-1FeCl3��Һ | |
b | 50 mL5%H2O2��Һ | ����Ũ���� | 1 mL0.1 mol��L-1FeCl3��Һ |
c | 50 mL5%H2O2��Һ | ����ŨNaOH��Һ | 1 mL 0.1 mol��L-1FeCl3��Һ |
d | 50 mL5%H2O2��Һ | MnO2 | |
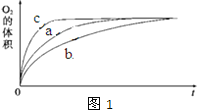
�� ���ʵ��a��b��c�����������������ʱ��仯�Ĺ�ϵ��ͼ1��ʾ���ɸ�ͼ�ܹ��ó���ʵ�������______________________��
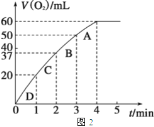
�� ���ʵ��d�ڱ�״���·ų������������ʱ��仯�Ĺ�ϵ��ͼ2��ʾ�����ͷ�Ӧ���ʱ仯��ԭ��________________������H2O2�ij�ʼ���ʵ���Ũ��Ϊ________________ (������λ��Ч����)��
���𰸡���1����![]() ��2H2O(g)=2H2(g)+2O2(g)��H=+482 kJ��mol-1��120.5
��2H2O(g)=2H2(g)+2O2(g)��H=+482 kJ��mol-1��120.5
��2����������������ʱ���ڼ��Ի����¼ӿ�H2O2�ķֽ����ʣ����Ի����¼���H2O2�ķֽ����������ŷ�Ӧ�Ľ���H2O2��Ũ�����٣�H2O2�ķֽ�����������0.11 mol/L
��������
�����������1����H2O�ǹ��ۻ��������ԭ�Ӻ���ԭ���γɹ��ۼ�������ʽΪ![]() ��
��
��ˮ�ֽ���������������������ʽΪ2H2O=2H2+O2����֪2molH2O��g���ֽ⣬����2mol������1mol�����������4molO-H���γ�1molO=O����2mol H-H�����������յ�����Ϊ4mol��463kJ/mol=1852kJ���ų�������Ϊ498kJ+2��436kJ=1370kJ���������յ�����Ϊ1852kJ-1370kJ=482kJ�������Ȼ�ѧ����ʽΪ2H2O(g)=2H2(g)+2O2(g) ��H=+482 kJ��mol-1��
��11.2L����״������H2Ϊ0.5mol��������0.5molˮ��������̬ˮ�ų�������Ϊ0.5mol��0.5��482KJ=120.5kJ��
��2����abc��Һ������Բ�ͬ����Ӧ�����ʲ�ͬ����ͼ���֪�ڼ��������·�Ӧ������������������·�Ӧ������С�����Եó��Ľ����������������䣬���Ի����ܼӿ�H2O2�ķֽ����ʣ����Ի����ܼ���H2O2�ķֽ����ʣ�
����ͼ���֪������б����С��˵����Ӧ������С��ԭ�������ŷ�Ӧ�Ľ���H2O2��Ũ�����٣�H2O2�ķֽ���������������ͼ���֪��������������60mL�����ʵ�����![]() �����Ը��ݷ���ʽ��֪˫��ˮ�����ʵ�����
�����Ը��ݷ���ʽ��֪˫��ˮ�����ʵ�����![]() ����˫��ˮ��Ũ����
����˫��ˮ��Ũ����![]() 0.11mol/L��
0.11mol/L��