��Ŀ����
3������ʵ�������ȷ���ǣ�������| A�� | ������ƿ�м�ˮʱ�������ڲ����������ζ�����װҺʱ��������©�� | |
| B�� | �����η�Һ©������ȡʵ��ʱ����©�÷�Һ©���Ͽڵķ������©����ƿһ������©�·������ķ������©�ζ���һ�� | |
| C�� | ����ˮ��pH�����ò�����պȡ��ˮ����pH��ֽ�ϣ������ɫ�������ɫ���Ƚ� | |
| D�� | ��������ʱ��������������ʯ�����ϣ��þƾ��Ƶ�������ȣ�ˮԡ���ȿ��Բ����¶ȼ� |
���� A�����ζ�����װҺʱ��Ҫ�ò�����������
B������ƿ���Ƚ�����ƿϴ����װ��ˮ���������ӣ����ã�����©ˮ���ٽ�����ƿ����������������ת180�ȣ��ٵ��ã�����©ˮ������ƿ��ã��ζ��ܣ��ر�����Ŀ��أ�װ��Һ�����ϸ��ӣ���Һ�岻��������ζ��ܲ�©ˮ��
C����ˮ�к��д��������Ư���ԣ�
D���������ֱ�Ӽ��ȣ�
��� �⣺A�����ζ�����װҺʱ��Ҫ�ò�����������������©������A����
B����Һ©�������Ӻͻ����ļ�©����������ƿ���Ӻ͵ζ��ܻ�������ͬ�ģ���B��ȷ��
C����ˮ�к��д��������Ư���ԣ�PH��ɫ��������ɫ����C����
D���������ֱ�Ӽ��ȣ���������ʱ���ɽ������������Ȧ�ϣ��þƾ��Ƶ�������ȣ���D����
��ѡB��
���� ���⿼�黯ѧʵ�鷽�������ۣ�Ϊ��Ƶ���㣬����ʵ�����������ʵ��ԭ���Ŀ��飬ע��װ�õ����ü�ʵ��IJ����ԡ������Է�������Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
14�����ֽⷴӦ����ѧ��ѧ�г�����һ�ַ�Ӧ���ͣ�
��ش��������⣮
��1����֪�ڳ����²��Ũ�Ⱦ�Ϊ0.1mol•L-1������6����Һ��pH��
���ֽⷴӦ������һ�����ɣ�һ�ֽ�ǿ������һ�ֽ�������ο��Է��ط�Ӧ�����ɽ�����
�ͽ�ǿ����Σ��磺2CH3COOH+Na2CO3�T2CH3COONa+CO2��+H2O���������Ƕȿ�����ͬʱ��ʾ����һ��
���ɣ����Խ�ǿ�����ʷ������Ʒ�Ӧ�������ɼ��Խ��������ʣ����ոù��ɣ����ж����з�Ӧ���ܷ�������AD�����ţ���
A��CO2+H2O+2NaClO��Na2CO3+2HClO
B��CO2+H2O+NaClO��NaHCO3+HClO
C��CO2+H2O+C6H5ONa��NaHCO3+C6H5OH
D��CO2+H2O+2C6H5ONa��Na2CO3+2C6H5OH
E��Na2CO3+C6H5OH��NaHCO3+C6H5ONa
��2���ø���ǰ����Ϣ�жϣ������£�Ũ�Ⱦ�Ϊ0.05mol/L������5�����ʵ���Һ�У�pH��С���Ǣݣ����ţ�����pH=1������ֵ����pH�����Ǣ٣����ţ�
��C6H5OH��CH3COOH��HClO4��HClO��H2SO4
��3��һЩ���ֽⷴӦ�ķ�������ѭ�����Ĺ��ɣ�����ת�������ڸ��ֽⷴӦ��
�ٹ�ҵ�Ͻ�ʯ�����봿����Һ��Ͽ��Ƶÿ��Ե�����Һ��
�ں����Ƽ�У���̼�������Һ�м��뱥��ʳ��ˮ�ɻ��С�մ��壮
�����������Ӧ���ܽ�����ֽⷴӦ����һ���ɣ����ֽⷴӦ���������ɸ��������ʵķ�����У�
��4�����ݣ�3���н��ۣ��ֽ�KI��Һ��AgCl�����Ͻ��裬����Թ۲쵽�������ǰ�ɫ�����Ϊ��ɫ����Ӧ�����ӷ���ʽΪI-+AgCl=AgI+Cl-��
��ش��������⣮
��1����֪�ڳ����²��Ũ�Ⱦ�Ϊ0.1mol•L-1������6����Һ��pH��
| ��Һ | CH3COONa | Na2CO3 | Na2CO3 | HClO | C6H5ONa |
| PH | 8.8 | 9.7 | 11.6 | 10.3 | 11.3 |
�ͽ�ǿ����Σ��磺2CH3COOH+Na2CO3�T2CH3COONa+CO2��+H2O���������Ƕȿ�����ͬʱ��ʾ����һ��
���ɣ����Խ�ǿ�����ʷ������Ʒ�Ӧ�������ɼ��Խ��������ʣ����ոù��ɣ����ж����з�Ӧ���ܷ�������AD�����ţ���
A��CO2+H2O+2NaClO��Na2CO3+2HClO
B��CO2+H2O+NaClO��NaHCO3+HClO
C��CO2+H2O+C6H5ONa��NaHCO3+C6H5OH
D��CO2+H2O+2C6H5ONa��Na2CO3+2C6H5OH
E��Na2CO3+C6H5OH��NaHCO3+C6H5ONa
��2���ø���ǰ����Ϣ�жϣ������£�Ũ�Ⱦ�Ϊ0.05mol/L������5�����ʵ���Һ�У�pH��С���Ǣݣ����ţ�����pH=1������ֵ����pH�����Ǣ٣����ţ�
��C6H5OH��CH3COOH��HClO4��HClO��H2SO4
��3��һЩ���ֽⷴӦ�ķ�������ѭ�����Ĺ��ɣ�����ת�������ڸ��ֽⷴӦ��
�ٹ�ҵ�Ͻ�ʯ�����봿����Һ��Ͽ��Ƶÿ��Ե�����Һ��
�ں����Ƽ�У���̼�������Һ�м��뱥��ʳ��ˮ�ɻ��С�մ��壮
�����������Ӧ���ܽ�����ֽⷴӦ����һ���ɣ����ֽⷴӦ���������ɸ��������ʵķ�����У�
��4�����ݣ�3���н��ۣ��ֽ�KI��Һ��AgCl�����Ͻ��裬����Թ۲쵽�������ǰ�ɫ�����Ϊ��ɫ����Ӧ�����ӷ���ʽΪI-+AgCl=AgI+Cl-��
11������������ȷ���ǣ�������
| A�� | ��AB2�͵Ĺ��ۻ����������ԭ��A������sp�ӻ�����ɼ� | |
| B�� | 25��ʱ����Mg��OH��2������Һ�м���������NH4Cl���壬c��Mg2+������ | |
| C�� | ��Ӧ2A��g��+B��g���T3C��s��+D��g����һ�����������Է����У�˵���÷�Ӧ�ġ�H��0 | |
| D�� | SO2��SiO2�ľ����У���ѧ������;������;���ͬ |
18���ҹ��ӹ������ijԭ�Ͼ��ⶨ��Ҫ����A��B��C��D��E����ǰ������Ԫ�أ���ԭ��������������Ԫ��A��B��C��D��E��ԭ�ӽṹ����Ϣ���£�
��ش��������⣺����A��B��C��D��E����Ӧ��Ԫ�ط��Ż��Ӧ������ѧʽ����
��1��Ԫ��E�����ڱ��е�λ��Ϊ�������ڵ�IB�壮
��2��д��A2D2���ӵĵ���ʽ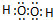 ��
��
��3��A��C��D��ԭ�Ӹ�����4��2��3�γɵĻ������ˮ��Һ��pH��7�����������������=������ԭ����NH4++H2O?NH3•H2O+H+�������ӷ���ʽ��ʾ����
��4��CD3-���ӵĿռ乹��Ϊƽ�������Σ�B2A6��C2A4�����о�����18�����ӣ����ǵķе����ϴ���Ҫԭ����N2H4����֮����������
��5��������C����̬�⻯��ͨ��E����������Һ�У���Ӧ������һ����ɽ�Ϊ���ӵ����ʣ��仯ѧʽΪ[Cu��NH3��4]SO4���û������д��ڵĻ�ѧ��������ABC������ĸ����
A�����Ӽ�B�����ۼ�C����λ��D����������
| Ԫ�� | ԭɫ���ʻ�ԭ�ӽṹ |
| A | ���ڱ���ԭ�Ӱ뾶��С��Ԫ�� |
| B | ԭ�Ӻ��������ֲ�ͬ��ԭ�ӹ���Ҹ���ԭ�ӹ�������ĵ�������ͬ |
| C | �����p�������� |
| D | λ�ڶ����ڣ���ԭ�ӵĺ���ɶԵ�������δ�ɶԵ�������3�� |
| E | λ��ds����ԭ�ӵ�������������A����ͬ |
��1��Ԫ��E�����ڱ��е�λ��Ϊ�������ڵ�IB�壮
��2��д��A2D2���ӵĵ���ʽ
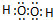 ��
����3��A��C��D��ԭ�Ӹ�����4��2��3�γɵĻ������ˮ��Һ��pH��7�����������������=������ԭ����NH4++H2O?NH3•H2O+H+�������ӷ���ʽ��ʾ����
��4��CD3-���ӵĿռ乹��Ϊƽ�������Σ�B2A6��C2A4�����о�����18�����ӣ����ǵķе����ϴ���Ҫԭ����N2H4����֮����������
��5��������C����̬�⻯��ͨ��E����������Һ�У���Ӧ������һ����ɽ�Ϊ���ӵ����ʣ��仯ѧʽΪ[Cu��NH3��4]SO4���û������д��ڵĻ�ѧ��������ABC������ĸ����
A�����Ӽ�B�����ۼ�C����λ��D����������
15��A��B��C��D��E��ԭ���������ε���������Ԫ�أ�ԭ������Ϊ5����������Ȼ����������˵����ȷ���ǣ�������
| A�� | BԪ�ص�����ϼ�Ϊ+4��ʱ���为���ϼ�Ҳ����Ϊ-3�� | |
| B�� | A��OH��n��HnEOm���ܷ�Ӧ | |
| C�� | HnCOmΪǿ��ʱ��HxDOyһ��Ϊǿ�� | |
| D�� | HnDOmΪǿ��ʱ��E�ķǽ�����һ����ǿ |
13����ͼ��C��D��E��F��X��Y���Ƕ��Ե缫������Դ��ͨ�����ң��е����̪��Һ���� F�������Ժ�ɫ��������˵����ȷ���ǣ�������
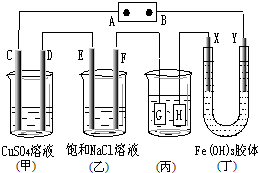

| A�� | �������顢����ȼ�ϵ������Դ�������ΪKOH��Һ����B���ĵ缫��ӦʽΪ��02+2H20+4e-�T40H- | |
| B�� | ���ã�����װ�ø�ͭ������HӦ����Ag�����Һ��AgNO3��Һ | |
| C�� | ������װ����Y���������ɫ���˵��������������������� | |
| D�� | C��D��E��F�缫���е������ɣ������ʵ�����Ϊ1��2��2��2 |



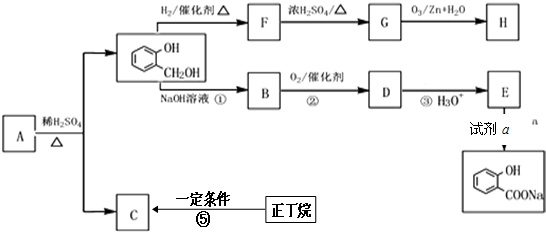
 ����F��G�ķ�Ӧ����Ϊ��ȥ��Ӧ��
����F��G�ķ�Ӧ����Ϊ��ȥ��Ӧ��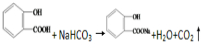 ��
��
 �ķ�Ӧ�Ƿ������ȫ�����һ����ʵ�����֤����д��ʵ������������ۣ�ȡ������Ӧ�����Һ���Թ��У������Ȼ�����Һ������Һ����ɫ����Ӧ����ȫ����Ӧ����ʣ�࣬��֮����ȫ��Ӧ��
�ķ�Ӧ�Ƿ������ȫ�����һ����ʵ�����֤����д��ʵ������������ۣ�ȡ������Ӧ�����Һ���Թ��У������Ȼ�����Һ������Һ����ɫ����Ӧ����ȫ����Ӧ����ʣ�࣬��֮����ȫ��Ӧ��


