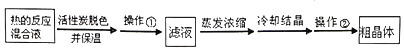
��Ŀ����
����Ŀ����.(1)��ˮ��100 ��ʱ��pH=6�����¶���0.1mol��L-1��NaOH��Һ�У�pH= _____����ˮ�������c(OH-)= ___mol��L-1��
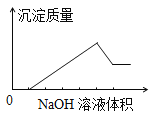
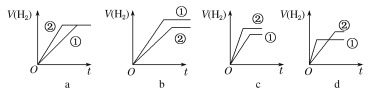
(2)�����£���pH��ͬ�������ͬ�Ĵ��������������Һ�ֱ���������п�۷�����Ӧ�����й����������(V)��ʱ��(t)�仯��ʾ��ͼ��ȷ����________(����ĸ)��(�ٱ�ʾ���ᣬ�ڱ�ʾ����)
(3)����ƽ�ⳣ���Ǻ���������ʵ���̶�ǿ������������
��ѧʽ | ����ƽ�ⳣ��(25 ��) |
HCN | K=4.9��10-10 |
CH3COOH | K=1.8��10-5 |
H2CO3 | K1=4.3��10-7��K2=5.6��10-11 |
��֪��25 ��ʱ����Ũ�ȵ�HCN��Һ��H2CO3��Һ��CH3COOH��Һ��������Һ��pH�ɴ�С��˳��Ϊ____________
(4)����ʱ��pH=4�������pH=9������������Һ��ϣ������û����Һ��pH=7�������������������Һ�������Ϊ_______��
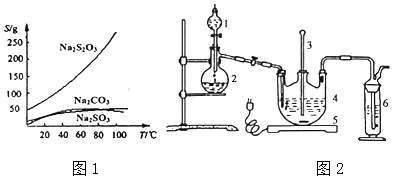
��.ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ����ʵ�����н�������ʵ�飺�����������գ�
ʵ���� | NaOH��Һ��Ũ��(mol/L) | �ζ����ʱ��NaOH��Һ��������(mL) | ����������Һ�����(mL) |
1 | 0.1000 | 15.12 | 20.00 |
2 | 0.1000 | 14.98 | 20.00 |
3 | 0.1000 | 14.90 | 20.00 |
(1)����10mL 0 .1000mol/L NaOH����Һ��
(2)ȡ20.00mL����ϡ������Һ������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶı�NaOH��Һ���еζ����ظ������ζ�����2��3�Σ���¼�������¡�
�ٵζ��ﵽ�յ��������______________________��
�ڸ����������ݣ��ɼ�����������Ũ��ԼΪ______________(������λ��Ч����)��
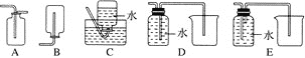
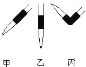
����ȥ��ʽ�ζ��������ݷ�����ͼ��ʾ��Ӧ���ò���____________��Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��
��������ʵ���У����в���(����������ȷ)�ֱ�Բⶨ������ʲôӰ�죿(����ƫ��������ƫ����������Ӱ����)
A���ζ��յ����ʱ���Ӷ���___________��
B����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ_______________��
���𰸡�11 10-11 c CH3COOH > H2CO3 > HCN 1:10 ��Һ��Ϊ�ۺ죬�ұ��ְ���Ӳ���ɫ 0.075mol/L �� ƫ�� ƫ��
��������
��.(1)��ˮ��100��ʱ��pH=6����˵�����¶���ˮ�����ӻ�������10-12����˸��¶���0.1mol��L-1��NaOH��Һ�У�������Ũ����10-11mol/L������Һ��pH=11����ˮ�������c(OH-)= 10-11mol��L-1��
(2)��������п��Ӧʱ����������������������ʵ��������ȣ�pH��ȵĴ�������ᣬ����Ũ�ȴ������ᣬ����pH�������ȵĴ�������ᣬ��������ʵ����������ᣬ��ֱ���������п��Ӧ����ų��������࣬�Ҵ���������������ʸ��죻��ѡc��
(3)����ĵ���ƽ�ⳣ��ԽС������Խ����pHԽ��25 ��ʱ����Ũ�ȵ�HCN��Һ��H2CO3��Һ��CH3COOH��Һ��������Һ��pH�ɴ�С��˳��ΪCH3COOH > H2CO3 > HCN��
(4)����������Ϊa L��NaOH��Һ�����Ϊb L��������c(H+)=10-4mol��L-1��NaOH��Һ��c(OH-)=10-5mol��L-1����Ϊ��Ϻ���ҺpH=7������a L��10-4mol��L-1=b L��10-5mol��L-1����a��b=1��10��
��. (1)�ټ������һ������������Һ����Һ����ɫǡ�ñ��dz��ɫ���Ұ�����ڲ���ɫ��
�ڸ���c(��)��V(��)=c(��)��V(��)����ҪV(NaOH)=![]() mL=15.00mL����������Ũ��Ϊ
mL=15.00mL����������Ũ��Ϊ![]() =0.075mol/L��
=0.075mol/L��
�ۼ�ʽ�ζ��ܵ�����ͨ����Ƥ���ڣ�ֻҪ���ζ�����ͷ���ϣ�������Ƥ���еIJ�����Ϳ��Խ����ݳ��ų����ʴ�Ϊ������
�ܸ���c(����)=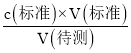 ������
������
A���ζ��յ����ʱ���Ӷ����������ı���Һ�����ƫ�ͣ������ҺŨ��ƫ�ͣ�
B����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ���ᵼ�²ⶨNaOH���ƫ�ⶨֵƫ�ߡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�