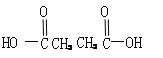��Ŀ����
����Ŀ�������£���ijһԪ��HA��Һ��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
ʵ���� | HA���ʵ���Ũ����mol��L��1�� | NaOH���ʵ���Ũ����mol��L��1�� | �����Һ��pH |
�� | 0.1 | 0.1 | pH��9 |
�� | c | 0.2 | pH��7 |
�� | 0.2 | 0.1 | pH<7 |
��1���ڢ���ʵ����0.1 mol��L��1HA��Һ��ˮ�ĵ���̶�Ϊa��0.1 mol��L��1NaOH��Һ��ˮ�ĵ���̶�Ϊb�����û����Һ��ˮ�ĵ���̶�Ϊc����a��b��c�ɴ�С��˳����_________��
��2���ڢ���ʵ����c_____0.2���<����������������
��3�����ݢ���ʵ�����õĻ����Һ������Һ������Ũ�ȴ�С����˳����____________��
��4�������£���֪KSP[Cu��OH��2]��2��10��20��ijCuSO4��Һ��c��Cu2������0.02 mol��L��1�����Ҫ����Cu��OH��2��������Ӧ������ҺpH����_________��
���𰸡���1��cab ��2���� ��3��c��A������c��Na������c��H������c��OH���� ��4��5
��������
�����������1����ʵ��ٵĽ�����������ǡ����ȫ��Ӧ�����Σ��Լ��ԣ�˵����Ϊ���ᣬ��ͬŨ�ȵ���ͼ���ˮ����̶��������д�����Һ����ˮ��ٽ�ˮ���룬����ˮ����̶ȵĹ�ϵΪcab����2�������ߵ�Ũ�ȵ�������ʱ��Һ��Ϊ���ԣ�������Ϊ���ԣ�˵��������������Ũ�ȴ��ڼ��Ũ�ȡ���3��ʵ����л�Ϻ�Ϊ��Ũ�ȵ�����ε���Һ����Һ�����ԣ�˵����ĵ���̶ȴ����ε�ˮ��̶ȣ�����ݵ���غ����������Ũ�ȹ�ϵΪc��A������c��Na������c��H������c��OH��������4��c��OH-��= ![]() mol/L������Һ��pH=5��
mol/L������Һ��pH=5��

 ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�����Ŀ���������ı仯�ͷ�Ӧ�Ŀ����ȽǶ��о���Ӧ������Ҫ������
��1����֪��Ӧ2H2+O2 =2H2OΪ���ȷ�Ӧ����ͼ����ȷ��ʾ�÷�Ӧ�������仯���� ��

��ѧ�� | H��H | O��O | H��O |
����kJ/mol | 436 | 496 | 463 |
�Ӷϼ��ͳɼ��ĽǶȷ���������Ӧ�������ı仯����ѧ���ļ�����������������1molˮ���Էų�����____________kJ
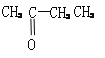
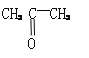
��2��ԭ��ؿɽ���ѧ��ת��Ϊ���ܡ���������ͬ��ͭ����п���õ������Ӻ����CuSO4��Һ�У���Ƴ�ԭ��أ����������� , �����ķ�ӦʽΪ ���������Һ��SO42�� ���� �����������������һ��ʱ���ȡ��ϴ�����������������������Ϊ12.9 g��������ͨ���ĵ��ӵ����ʵ����� mol��
��3��һ���¶��£���3 molA�����1mol B����ͨ��һ�ݻ��̶�Ϊ2L���ܱ������У��������·�Ӧ��3A(g)��B(g) ![]() xC(g)����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L����1min�ڣ�B��ƽ����Ӧ����Ϊ ��XΪ ������Ӧ��2min�ﵽƽ�⣬ƽ��ʱC��Ũ�� 0.8mol/L������ڣ�С�ڻ���ڡ�����
xC(g)����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L����1min�ڣ�B��ƽ����Ӧ����Ϊ ��XΪ ������Ӧ��2min�ﵽƽ�⣬ƽ��ʱC��Ũ�� 0.8mol/L������ڣ�С�ڻ���ڡ�����
����Ŀ����1���о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣 ��֪��
2SO2(g)+O2(g) 2SO3(g) ��H= -196.6kJ��mol��1
2NO(g)+O2(g)![]() 2NO2(g) ��H= -113.0kJ��mol-1
2NO2(g) ��H= -113.0kJ��mol-1
��ӦNO2(g)+SO2(g)![]() SO3(g)+NO(g)����H= kJ��mol-1
SO3(g)+NO(g)����H= kJ��mol-1
��2�����Ƕ�ֲ����������ȱ�ٵ�Ԫ�أ��ϳɰ��ķ�Ӧ����������ʳ�����Ӧ���£�
N2(g)��3H2(g)![]() 2NH3(g)��
2NH3(g)��
��һ�������£���һ������N2��H2�Ļ���������ij�����ܱ������У�һ��ʱ�������������˵���÷�Ӧ�ﵽƽ��״̬���� ������ţ���
A�������л��������ܶȲ���ʱ��仯 |
B������3 mol H-H����ͬʱ�γ�6 mol N-H�� |
C��N2��H2��NH3�����ʵ���֮��Ϊ1:3:2 |
D�������е�ѹǿ����ʱ��仯 |
��3����25 ����101 kPa�£�1 g�״�ȼ������CO2��Һ̬ˮʱ����22.68 kJ����÷�Ӧ���Ȼ�ѧ����ʽ��____________��