��Ŀ����
��1����֪25��ʱ�й�����ĵ���ƽ�ⳣ����
| ���ữѧʽ | HSCN | CH3COOH | HCN | H2CO3 |
| ����ƽ�ⳣ�� | 1.3��10��1 | 1.8��10��5 | 4.9��10��10 | K1=4.3��10��7 K2=5.6��10��11 |
�ٵ����ʵ���Ũ�ȵ�a.CH3COONa��b.NaCN��c.Na2CO3��d.NaHCO3��Һ��pH�ɴ�С��˳��Ϊ
������ţ���
��25��ʱ����20 mL 0.1 mol��L��1 CH3COOH��Һ��20 mL 0.1 mol��L��1HSCN��Һ�ֱ���20 mL 0.1 mol��L��1NaHCO3��Һ��ϣ�ʵ���ò��������������V����ʱ�䣨t���ı仯��ͼ��ʾ����Ӧ��ʼ��������Һ����CO2��������ʴ������Բ����ԭ���� ����Ӧ��������������Һ�У�c��CH3COO���� c��SCN�������������������������

���������¶Ȳ��䣬�ڴ�����Һ�м����������ᣬ���������С����______������ţ���
a. c(CH3COO��) b. c(H+) c. Kw d. �������ƽ�ⳣ��
��2����ͼΪij�¶��£�PbS��s����ZnS��s����FeS��s���ֱ�����Һ�дﵽ�����ܽ�ƽ�����Һ��S2��Ũ�ȡ�����������Ũ�ȱ仯�������������ֳ����м����ᣬ�����ܽ���� ���ѧʽ�����������ɵ�ZnS��Һ�е�����������ͬŨ�ȵ�Pb2+��Fe2+����Һ����ZnS������ת��Ϊ ���ѧʽ��������

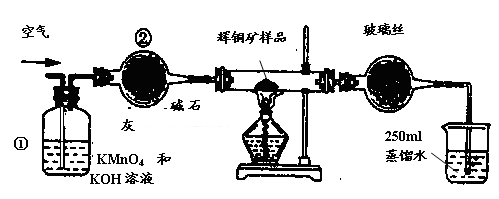
��3������ȼ�ϵ�ؿ����������������ʡ���ͼ�����ü���ȼ�ϵ�ص��50 mL 2 mol��L��1���Ȼ�ͭ��Һ��װ��ʾ��ͼ��

��ش�
�� ����ȼ�ϵ�صĸ�����Ӧʽ�� ��
�� ����·����0.1 mol����ͨ��ʱ�� ������________g
��13�֣���1���� c b d a ��2�֣�
��ͬŨ�ȵ�HSCN��CH3COOH����ǿ����NaHCO3��Һ��Ӧ�죨2�֣� ����1�֣� �� a��2�֣�
��2��FeS ��1�֣� PbS ��1�֣�
��3����CH4 ��8e�� + 2H2O��CO2 + 8H+ ��2�֣� ��b��1�֣� 3.2��1�֣�
���������������1���ٵ���ƽ�ⳣ��ԽС����Խ������˸��ݵ���ƽ�ⳣ����֪������ǿ��˳����HSCN��CH3COOH��H2CO3��HCN��HCO3������Խ������Ӧ������Խ����ˮ�⣬��Һ��pHԽ��������ʵ���Ũ�ȵ�a.CH3COONa��b.NaCN��c.Na2CO3��d.NaHCO3��Һ��pH�ɴ�С��˳��Ϊc��b��d��a��
�ڸ��ݵ��볣����֪��ͬŨ�ȵ�HSCN��CH3COOH����ǿ��������NaHCO3��Һ��Ӧ�죻��Ӧ������õ����Ǵ����ƺ�NaSCN��Һ������������HSCN��CH3COOH����ǿ�����Դ����Ƶ�ˮ��̶�ǿ��NaSCN��ˮ��̶ȣ������Һ��c��CH3COO������c��SCN������
�۸���ͼ���֪��PbS��ZnS��FeS���ܶȻ�������������������������ֳ����м����ᣬ�����ܽ����FeS�������ܽ�ƽ���֪���������������ɸ����ܵķ���ת��������������ɵ�ZnS��Һ�е�����������ͬŨ�ȵ�Pb2+��Fe2+����Һ����ZnS������ת��ΪPbS������
��3����ԭ����и���ʧȥ���ӣ������õ����ӡ�����ڸ�ȼ�ϵ���м����ڸ���ͨ�룬����������ͨ�롣���ڵ���������Ե���ʣ����Ը����缫��Ӧʽ��CH4 ��8e�� + 2H2O��CO2 + 8H+��
�ڸ���װ��ͼ��֪�����缫��ȼ�ϵ�صĸ�������������������Һ�е�ͭ�����������ŵ磬�缫��Ӧʽ��Cu2����2e����Cu�����Բ��缫��������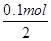 ��64g/mol��3.2g��
��64g/mol��3.2g��
���㣺����������ʵĵ��롢����ˮ�⡢��Һ������Ũ�ȴ�С�Ƚϡ��ܶȻ�������Ӧ���Լ��ܽ�ƽ�⡢�绯ѧԭ����Ӧ�úͼ����

ˮ�����ؽ���Ǧ����Ⱦ���ⱸ�ܹ�ע��ˮ��Һ��Ǧ�Ĵ�����̬��Ҫ��Pb2����Pb(OH)����Pb(OH)2��Pb(OH)3-��Pb(OH)42-������̬��Ũ�ȷ���������ҺpH�仯�Ĺ�ϵ����ͼ��ʾ�� 
(1)Pb(NO3)2��Һ�У� ________2(�>����������<��)��������Һ�е����Ȼ����Һ��
________2(�>����������<��)��������Һ�е����Ȼ����Һ�� ���ӣ����ܵ�ԭ����________________________________��
���ӣ����ܵ�ԭ����________________________________��
(2)��Pb(NO3)2��Һ�е���ϡNaOH��Һ��pH��8ʱ��Һ�д��ڵ�������(Na������)��__________��pH��9ʱ��Ҫ��Ӧ�����ӷ���ʽΪ_______________________��
(3)ij�������Ʊ���һ��������Ǧ��������Чȥ��ˮ�еĺ���Ǧ��ʵ�������±���
| ���� | Pb2�� | Ca2�� | Fe3�� | Mn2�� | Cl�� |
| ����ǰŨ��/(mg��L��1) | 0.100 | 29.8 | 0.120 | 0.087 | 51.9 |
| ������Ũ��/(mg��L��1) | 0.004 | 22.6 | 0.040 | 0.053 | 49.8 |
(4)�������Ǧ��(��EH��ʾ)��Ǧ��Ҫ�����ķ�Ӧ������Ϊ��2EH(s)��Pb2��
 E2Pb(s)��2H������Ǧ�������pH��ΧΪ( )
E2Pb(s)��2H������Ǧ�������pH��ΧΪ( )A��4��5 B��6��7 C��9��10 D��11��12
��1���֣�
���ռ�������ˮ�����ξ��ơ���һ�ξ�����Ҫ���ó�������ȥ����ˮ��Ca2+��Mg2+��Fe3+��SO42-�����ӣ��������£�
��. �����ˮ�м������BaCl2��Һ�����ˣ�
��. ��������Һ�м������Na2CO3��Һ�����ˣ�
��. ��Һ���������pH�����һ�ξ�����ˮ��
��1�����̢��ȥ��������______��
��2�����̢����ɵIJ��ֳ��������ܽ�ȣ�20��/g�����±���
| CaSO4 | Mg2(OH)2CO3 | CaCO3 | BaSO4 | BaCO3 |
| 2.6��10-2 | 2.5��10-4 | 7.8��10-4 | 2.4��10-4 | 1.7��10-3 |
�� ���̢�ѡ��BaCl2����ѡ��CaCl2�����ñ������ݽ���ԭ��______��
�� ��ȥMg2+�����ӷ���ʽ��______��
�� ���Ca2+��Mg2+��Ba2+�Ƿ����ʱ��ֻ����Ba2+���ɣ�ԭ����_____��
��3���ڶ��ξ���Ҫ��ȥ����I-��IO3-��NH4+��Ca2+��Mg2+������ʾ�����£�

�� ���̢���ȥ��������______��
�� ��ˮb�к���SO42-��Na2S2O3��IO3- ��ԭΪI2�����ӷ���ʽ��______��
�� ����VI�У��ڵ��۵�����������NaOH����ϻ�ѧƽ��ԭ�����ͣ�_______��
��ҵ��Ϊ�˲ⶨ��ͭ����Ҫ�ɷ���Cu2S����Cu2S�������������������ͼװ�á�ʵ��ʱ�����²��������
| A������ȫ��������ʹ���Ϊ��ͼװ�ã������װ�õ������ԡ� |
| B����ȡ��ϸ�Ļ�ͭ����Ʒ1.000g�� |
| C���������õ���ƷС�ĵط���Ӳ�ʲ������С� |
| D����ÿ����1L�����ʹ�������� |
F. ��ȡ25.00ml��SO2��ˮ��Һ��250ml��ƿ�У���0.0100mol/L KMnO4����Һ�ζ����յ㡣���������������ظ��ζ�2��3�Ρ�
�Իش��������⣺
��1��װ�âٵ�������_________________��װ�âڵ�������____________________��
��2���ٶ���ͭ���е���ȫ��ת��ΪSO2������ȫ����ˮ���գ������F����������Ӧ�Ļ�ѧ����ʽΪ ��������_______________________________������ʱ���жϵζ��Ѿ��ﵽ�յ㡣
��3��������F�ĵζ�������±���ʾ�����ͭ����Ʒ��Cu2S������������_________��
| �ζ� ���� | ������Һ�� ���/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.04 | 21.03 |
| 2 | 25.00 | 1.98 | 21.99 |
| 3 | 25.00 | 3.20 | 21.24 |
��4���������������һ�����Ե�ȱ��Ӱ���˲ⶨ����������ڲ���ʧ������Ϊ�� ��дһ�ּȿɣ���
��5����֪�ڳ�����FeS �� Ksp�� 6 . 25 �� 10 ��18, H2S ������Һ�� c (H������ c (S2����֮��������¹�ϵ�� c2 (H��) ��c��S2��) =" 1" . 0��10��22���ڸ��¶��£������� FeS Ͷ�����ⱥ����Һ�У���ʹ��Һ��c��Fe2+��Ϊ lmol/L��Ӧ������Һ��c��Hʮ��Ϊ__________������
ʵ������NaOH��������0.1 000mol��L-1NaOH��Һ500mL��
��1����������ƽ��ȡNaOH����________g����Һ���ƹ����õ����в������������״�ʹ�õ��Ⱥ�˳��������________ (������ѡ�����)
A�������� B����ͷ�ι� C���ձ� D��500mL����ƿ
��2���������Ƶ�0.1000mol��L-1NaOH��Һͨ���к͵ζ��ⶨһԪ����HA��ҺŨ�ȣ�ÿ�εζ�ȡ�õ�HA��Һ��Ϊ20.00mL��ʹ�÷�̪��ҺΪָʾ�����ζ��յ�ı�־��____________________________���ζ���ʵ�����ݼ�¼��
| �ζ����� | NaOH��Һ�����mL�� | |
| V1 | V2 | |
| 1 | 3.05 | 44 |
| 2 | 1.45 | 41.5 |
| 3 | 7.65 | 47.6 |
��������ʵ�����ݣ���ø�HA��Һ���ʵ���Ũ��Ϊ____________________��
��3�������к͵ζ�ʵ���У����²������ܵ���������ҺŨ��ƫ�ߵ���__________(��ѡ�����)
A���ζ����ô�װҺ��ϴ
B����ƿ�ô�װҺ��ϴ
C���ζ�ǰ�ζ���ĩ������û�Ͼ�
D���ζ�ǰƽ�ӹ۲�������ζ����ӹ۲����
E�����õı�Һ(NaOH��Һ)Ũ��ƫ��
��4����pH�Ʋ�ø�HA��ҺpH=a����������к͵ζ����������ʵ���¶���HA��Ka=________��
����M��N�ɹ�����ͼ��ʾ��װ��.���з�����ȷ���� 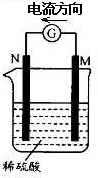
| A������������N>M |
| B��M���ܽ� |
| C��������ӦΪ2H++2e- =H2�� |
| D��SO42-��M�������ƶ� |
 I��+ I2��ʵ���ҿ���ͨ��������ԭ�ζ����ⶨƽ��ʱI3����Ũ��
I��+ I2��ʵ���ҿ���ͨ��������ԭ�ζ����ⶨƽ��ʱI3����Ũ��