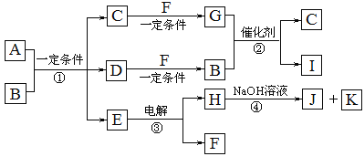
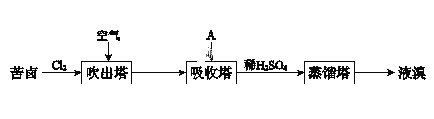
��Ŀ����
����Ŀ��������ë�⡱��һ���̲裬�̲��к��еĵ�����(��ѧʽΪC76XnO46)��������Ѫѹ���������Ƚⶾ�ȹ�Ч���ش��������⣺
(1)��֪��������ȫȼ��ʱ�õ�CO2��H2O����X����______Ԫ�أ��������Ħ��������1700g��L��1����n=______��170g��������ȫȼ��ʱ�ɵõ�______gH2O��
(2)��170g������ij��ȼ��ʱ������3.6molCO2�������ɵ�CO���______g����������»ָ������£����û�������ƽ��Ħ������Ϊ______(����1λС��)��
���𰸡�H(����) 52 46.8 112 35.6g��mol��1
��������
(1)ȼ�ղ����к���C��H��O����Ԫ�أ���ȼ��ʱ���������⣬ֻ�ṩ��Ԫ�أ��ɴ˿�ȷ��������������Ԫ�أ��������Ħ��������1700g��L��1�����õ���������и�ԭ�ӵ����ԭ��������Ϊ1700�������n�����170g����������ʵ�����������Ԫ���غ㣬�������ȫȼ��ʱ����H2O��������
(2)�����170g������������̼ԭ�ӵ����ʵ���������̼ԭ���غ㣬���������CO�����ʵ������Ӷ������������������»ָ������£�ˮת��ΪҺ�壬��CO2��CO�����������ʵ�������������û�������ƽ��Ħ��������
(1)ȼ�ղ����к���C��H��O����Ԫ�أ�����ȼ��ΪO2�����Ե�������һ��������Ԫ�أ��Ӷ��ó�X����H(����)Ԫ�أ��������Ħ��������1700g��L��1����76��12+1��n+16��46=1700��n=52��170g���������ʵ���Ϊ![]() =0.1mol����ȫȼ��ʱ�ɵõ�H2O 0.1mol��26��18g/mol= 46.8g����Ϊ��H(����)��52��46.8��
=0.1mol����ȫȼ��ʱ�ɵõ�H2O 0.1mol��26��18g/mol= 46.8g����Ϊ��H(����)��52��46.8��
(2)170g�������У�n(C)=![]() =7.6mol��ij��ȼ��ʱ������3.6molCO2�������ɵ�CO�����ʵ������Ϊ7.6mol-3.6mol=4mol������Ϊ4mol��28g/mol=112g����������»ָ������£����û�������ƽ��Ħ������Ϊ
=7.6mol��ij��ȼ��ʱ������3.6molCO2�������ɵ�CO�����ʵ������Ϊ7.6mol-3.6mol=4mol������Ϊ4mol��28g/mol=112g����������»ָ������£����û�������ƽ��Ħ������Ϊ![]() =35.6g��mol��1����Ϊ��112��35.6g��mol��1��
=35.6g��mol��1������112��35.6g��mol��1��

 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д�����Ŀ��PH3����ʳɱ�洦��ʱ���õ�Ѭ��ɱ�����ˮú���任ʱ������PH3��ʹ�����ж��������ѳ����ش��������⣺
��1��PH3ͨ��NaClO��Һ�ѳ�PH3ʱ������������һ�ֺ������ҷ�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ4��1����ú�����Ļ�ѧʽΪ______��
��2����֪���м������ݼ�P4�����ף����ӽṹ��
��ѧ�� | P-P | H-H | P-H |
|
����/��kJmol-1�� | 213 | 436 | 322 |
��Ӧ4PH3��g��P4��g��+6H2��g����H=______kJmol-1��ij�¶�ʱƽ����ϵ��c��PH3��=0.25molL-1��c��H2��=c��P4��=0.50molL-1����ƽ�ⳣ��K=______��
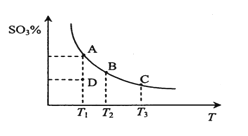
��3�����ױ�������Ӧ6.25CO2��g��+Fe3O4��s��+3PH3��g��=3FePO4��s��+4.5H2O��g��+6.25C��s����������ý�ж���������Ӧ����ƽ�ⳣ��Kp��KpΪ�Է�ѹ��ʾ��ƽ�ⳣ�����Ķ���ֵ���¶ȵĹ�ϵ��ͼ��ʾ��
�ٸ÷�Ӧ����H______0���������������=������
��ͼ��lgKp=______[�г��÷�ѹp��CO2����p��PH3����p��H2O����ʾ�ļ���ʽ]��
��4����Ӧ��CH3��3AuPH3����CH3��AuPH3+C2H6���������£�
��һ������CH3��3AuPH3![]() ��CH3��3Au+PH3���췴Ӧ��
��CH3��3Au+PH3���췴Ӧ��
�ڶ�������CH3��3Au![]() C2H6+CH3Au������Ӧ��
C2H6+CH3Au������Ӧ��
��������CH3Au+PH3![]() ��CH3��AuPH3���췴Ӧ��
��CH3��AuPH3���췴Ӧ��
�ٷ�Ӧ���м������PH3��______��
�ڵ�______�����һ������������������Ӧ�Ļ�����
��5����Cu2+��Pd2+Һ���ѳ�PH3�ķ�ӦΪ��PH3+2O2 H3PO4������������ͬʱ�ܽ�����Һ��O2�����������PH3�ľ���Ч����ʱ��Ĺ�ϵ��ͼ��ʾ��
H3PO4������������ͬʱ�ܽ�����Һ��O2�����������PH3�ľ���Ч����ʱ��Ĺ�ϵ��ͼ��ʾ��
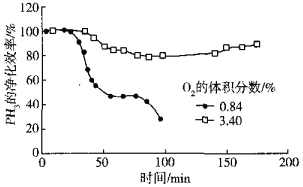
O2�����������PH3�ľ���Ч�ʸߵ�ԭ����______������ײ���۵�˵������