��Ŀ����
16����1��AԪ��ԭ�Ӻ���M���������L���������һ�룬��д��AԪ�ػ�̬ʱ��������Ų�ʽ��1s22s22p63s23p2����2��Bԭ�ӵ����������Ų�ʽΪnsnnp2n����BԪ����Ԫ�����ڱ�����λ��Ϊ�ڶ�����VIA�壮
��3�����dz�����ͬһԭ�ӹ�����˶��ģ����������෴��2�����ӣ���Ϊ�����Ӷԡ�������ͬһԭ�ӹ�����˶��ĵ������ӣ���Ϊ��δ�ɶԵ��ӡ�����ô��ԭ�ӵġ�δ�ɶԵ��ӡ���2����
���� ��1��AԪ��ԭ�Ӻ���M���������L���������һ�룬��Aԭ�Ӻ�����14�����ӣ�ΪSiԪ�أ�
��2��Bԭ�ӵ����������Ų�Ϊnsnnp2n������s�ܼ��������2�����ӣ���n=2��Bԭ���������Ų�Ϊ2s22p4����BΪOԪ�أ�
��3����ԭ�ӵĵ����Ų�ʽΪ��1s22s22p63s23p4���ݴ��жϣ�
��� �⣺��1��AԪ��ԭ�Ӻ���M���������L���������һ�룬��Aԭ�Ӻ�����14�����ӣ�ΪSiԪ�أ����̬ԭ�ӵ����Ų�ʽ1s22s22p63s23p2��
�ʴ�Ϊ1s22s22p63s23p2��
��2��Bԭ�ӵ����������Ų�Ϊnsnnp2n������s�ܼ��������2�����ӣ���n=2��Bԭ���������Ų�Ϊ2s22p4����BΪOԪ�أ�����Ϊ�ڶ�����VIA�壬�ʴ�Ϊ������VIA��
��3����ԭ�ӵĵ����Ų�ʽΪ��1s22s22p63s23p4������ԭ�ӵ�δ�ɶԵ�����Ϊ2���ʴ�Ϊ��2��
���� ���⿼���˺�������Ų����ɡ�Ԫ�������ڱ�λ�õ��жϣ��ѶȲ��������������Ų������ǹؼ���ע������Ų�ʽ������Ų�ͼ������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
6����֪�������£�Ksp��AgCl��=1.8��10-10��Ksp��AgI��=8.3��10-17��������������ȷ���ǣ�������
| A�� | ��AgCl������Һ�м���KI��Һ�������ɰ�ɫת��Ϊ��ɫ | |
| B�� | ��AgCl�ı�����Һ�м���KCl���壬��AgCl��������Һ��c��Ag+��=c��Cl-�� | |
| C�� | �����£�AgCl�ڱ���KCl��Һ�е�Ksp���ڴ�ˮ�е�KspС | |
| D�� | ��0.001��mol•L-1��AgNO3��Һ����NaCl��NaI�Ļ����Һ�У�һ���Ȳ���AgI���� |
7������ȥ���������л�����������ʣ�����������Ϊ���ʣ������ܴﵽĿ���ǣ�������
| A�� | �������������ᣩ���ӱ���Na2CO3 ��Һ��������ú�Һ | |
| B�� | �Ҵ���ˮ��������������ʯ�ң����� | |
| C�� | ���ᣨ�Ҵ�������������ƣ����� | |
| D�� | ����Һ�壩������NaOH��Һ����Һ |
4��Ԫ�����ڱ���ѧϰ��ѧ����Ҫ���ߣ���������������Ϣ���ɣ������ǰ��ֶ�����Ԫ�ص������Ϣ����֪Be��ԭ�Ӱ뾶Ϊ0.089nm��
Fԭ���������ӣ�G�������Ϊ���۾���ֵ��ȣ��������������Ǵ����Ķ�����HԪ�ص�����ɫ��Ӧ�ʻ�ɫ��
��1��BԪ����Ԫ�����ڱ��е�λ�õ������ڢ�A�壮B�γɵļ����ӵĽṹʾ��ͼ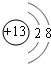 ��
��
��2����������Ԫ�ص�����������Ӧ��ˮ������������ǿ����HClO4���ѧʽ����
��3���õ���ʽ��ʾC��H�γɻ�����Ĺ��� ��
��
��4��H��E�γ�ԭ�Ӹ�����Ϊ1��1�Ļ�������������ѧ������Ϊ���Ӽ������ۼ���
��5��F��G�γɵ���Ļ�����ĵ���ʽ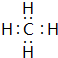 ��
��
��6��E���⻯���C���⻯����۷е�ߵ�ԭ����ˮ����֮�����������������֮��Ϊ���»���������ȷ��»�����ǿ��
��7��A��B��C��E���γɵļ����Ӱ뾶�ɴ�С��˳��ΪS2-��O2-��Mg2+��Al3+��
| Ԫ�ش��� | A | B | C | D | E |
| ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.102 | 0.099 | 0.074 |
| ��Ҫ���ϼ� | +2 | +3 | +6��-2 | -1 | -2 |
��1��BԪ����Ԫ�����ڱ��е�λ�õ������ڢ�A�壮B�γɵļ����ӵĽṹʾ��ͼ
 ��
����2����������Ԫ�ص�����������Ӧ��ˮ������������ǿ����HClO4���ѧʽ����
��3���õ���ʽ��ʾC��H�γɻ�����Ĺ���
 ��
����4��H��E�γ�ԭ�Ӹ�����Ϊ1��1�Ļ�������������ѧ������Ϊ���Ӽ������ۼ���
��5��F��G�γɵ���Ļ�����ĵ���ʽ
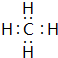 ��
����6��E���⻯���C���⻯����۷е�ߵ�ԭ����ˮ����֮�����������������֮��Ϊ���»���������ȷ��»�����ǿ��
��7��A��B��C��E���γɵļ����Ӱ뾶�ɴ�С��˳��ΪS2-��O2-��Mg2+��Al3+��
1������������֤��ij������������ʵ��ǣ�������
| A�� | ˮ��Һ�ĵ����������� | |
| B�� | ��Һ�е������������δ����ķ��ӹ��� | |
| C�� | �ۻ�ʱ������ | |
| D�� | �������ӻ�������ǹ��ۻ����� |
5��ijͬѧΪ����ij��Һ���Ƿ��г��������������ӣ���������ͼ��ʾ��ʵ����������м�������в�����������ʹʪ��ĺ�ɫʯ����ֽ�������ɸ�ʵ���ܵõ�����ȷ�����ǣ�������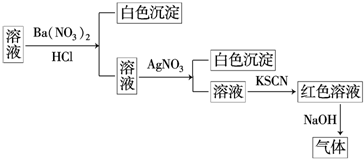

| A�� | ԭ��Һ��һ������SO42- | B�� | ԭ��Һ��һ������NH4+ | ||
| C�� | ԭ��Һ��һ������Cl- | D�� | ԭ��Һ��һ������Fe3+ |
6�����������У����зǼ��Թ��ۼ����ǣ�������
| A�� | ���� | B�� | �Ȼ��� | C�� | �������� | D�� | �Ȼ�� |
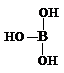 ������ѧ֪ʶ������ش��������⣺
������ѧ֪ʶ������ش��������⣺ ��
��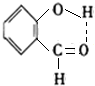 ��
��