ΧβΡΩΡΎ»ί
ΨέΚœΝρΥαΧζ”÷≥ΤΨέΧζΘ§Μ·―ß ΫΈΣ[Fe2(OH)n(SO4)3Θ≠0.5n]mΘ§ ΙψΖΚ”Ο”ΎΈέΥ°¥ΠάμΓΘ Β―ι “άϊ”ΟΝρΥα≥ß…’‘ϋ(÷ς“Σ≥…Ζ÷ΈΣΧζΒΡ―θΜ·ΈοΦΑ…ΌΝΩFeSΓΔSiO2Β»)÷Τ±ΗΨέΧζΚΆ¬ΧΖ·(FeSO4ΓΛ7H2O)Θ§Ιΐ≥Χ»γœ¬ΘΚ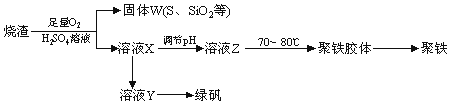
Θ®1Θ©―ι÷ΛΙΧΧεW±Κ…’Κσ≤ζ…ζΒΡΤχΧεΚ§”–SO2ΒΡΖΫΖ® «ΘΚ________________________ΓΘ
Θ®2Θ© Β―ι “÷Τ±ΗΓΔ ’Φ·Η…‘οΒΡSO2Θ§Υυ–η“«Τς»γœ¬ΓΘΉΑ÷ΟA≤ζ…ζSO2Θ§Α¥ΤχΝςΖΫœρΝ§Ϋ”Ης“«ΤςΫ”ΩΎΘ§Υ≥–ρΈΣaΓζ____Γζ____Γζ____Γζ____ΓζfΓΘ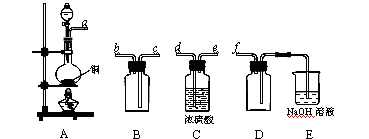
ΉΑ÷ΟA÷–ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ_________________________________________ΓΘ
Θ®3Θ©÷Τ±Η¬ΧΖ· ±Θ§œρ»ή“ΚX÷–Φ”»κΙΐΝΩ__________Θ§≥δΖ÷Ζ¥”ΠΚσΘ§Ψ≠Ιΐ¬Υ≤ΌΉςΒΟΒΫ»ή“ΚYΘ§‘ΌΨ≠≈®ΥθΓΔΫαΨßΒ»≤Ϋ÷ηΒΟΒΫ¬ΧΖ·ΓΘΙΐ¬ΥΥυ–ηΒΡ≤ΘΝß“«Τς”–_____________________ΓΘ
Θ®4Θ©”ϊ≤βΕ®»ή“ΚY÷–Fe2+ΒΡ≈®Ε»Θ§–η“Σ”Ο»ίΝΩΤΩ≈δ÷ΤKMnO4±ξΉΦ»ή“ΚΘ§”ΟKMnO4±ξΉΦ»ή“ΚΒΈΕ® ±”Π―Γ”Ο________ΒΈΕ®Ιή(ΧνΓΑΥα ΫΓ±ΜρΓΑΦν ΫΓ±)ΓΘ
Θ®5Θ©»ή“ΚZΒΡpH”ΑœλΨέΧζ÷–ΧζΒΡ÷ ΝΩΖ÷ ΐΓΘ»τ»ή“ΚZΒΡpHΤΪ–ΓΘ§ΫΪΒΦ÷¬ΨέΧζ÷–ΧζΒΡ÷ ΝΩΖ÷ ΐ______(ΧνΓΑΤΪ¥σΓ±ΓΔΓΑΤΪ–ΓΓ±ΜρΓΑΈό”ΑœλΓ±)ΓΘ
Θ®1Θ©ΫΪΤχΧεΆ®»κΤΖΚλ»ή“ΚΘ§»ή“ΚΆΥ…ΪΘ§Φ”»»Μ÷Η¥‘≠…ΪΘ®2Ζ÷Θ©
Θ®2Θ©d ΓζeΓζcΓζb Θ®2Ζ÷Θ©
Cu+2H2SO4(≈®)  CuSO4+SO2Γϋ+2H2O Θ®2Ζ÷Θ©
CuSO4+SO2Γϋ+2H2O Θ®2Ζ÷Θ©
Θ®3Θ©Χζ–Φ Θ®2Ζ÷Θ© …’±≠ΓΔ¬©ΕΖΓΔ≤ΘΝßΑτ Θ®2Ζ÷Θ©
Θ®4Θ©Υα Ϋ Θ®2Ζ÷Θ©
Θ®5Θ©ΤΪ–Γ Θ®2Ζ÷Θ©
ΫβΈω ‘ΧβΖ÷ΈωΘΚΘ®1Θ©”ΟΤΖΚλ»ή“ΚΦλ―ιSO2ΤχΧεΘ®2Θ©”Ο≈®ΝρΥαΗ…‘οSO2Θ§”Οœρ…œ≈≈Ω’Ζ® ’Φ·Θ”ΘœΘ≤ΘΜ Β―ι “”Ο≈®ΝρΥαΚΆΆ≠Ζ¥”Π÷Τ»ΓΘ”ΘœΘ≤ΘΜΘ®3Θ©≤Μ“ΐ»κ–¬ΒΡ‘”÷ Θ§Ω…”ΟΧζ–ΦΫΪΙΐΝΩΒΡœΓΝρΥα≥ΐ»ΞΘ®4Θ©ΗΏΟΧΥαΦΊΨΏ”–«Ω―θΜ·–‘Θ§Ι ”Π”ΟΥα ΫΒΈΕ®Ιή ΔΖ≈ Θ®5Θ©»τ»ή“ΚZΒΡpHΤΪ–ΓΘ§ΫΪΒΦ÷¬ΨέΧζ»ήΫβΘ§ ΙΧζΒΡ÷ ΝΩΖ÷ ΐΤΪ–ΓΓΘ
ΩΦΒψΘΚΩΦ≤ιΜ·―ß Β―ι

 ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ
ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ –Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ
–Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗΔώΘ°Θ®1Θ©“―÷ΣΡ≥”–ΜζΈοA÷ΜΚ§”–CΓΔHΓΔO»ΐ÷÷‘ΣΥΊΘ§Ά®Ιΐ‘ΣΥΊΖ÷Έω÷ΣΚ§ΧΦ54.55%Θ§Κ§«β9.10%ΓΘ÷ ΤΉΖ÷ΈωΤδœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ88Θ§Ψ≠ΚλΆβΙβΤΉΖ÷ΈωΤδ÷–÷ΜΚ§CΘ≠HΦϋΚΆ ΦϋΘ§ΤδΚΥ¥≈Ι≤’ώ«βΤΉΆΦœ‘ Ψ”–»ΐ÷ΊΖεΘ§ΖεΟφΜΐ÷°±»ΈΣ3ΘΚ2ΘΚ3Θ§ΗΟ”–ΜζΈο≤ΜΚ§”–CH3Θ≠OΘ≠Θ§‘ρAΒΡΫαΙΙΦρ ΫΈΣ
ΦϋΘ§ΤδΚΥ¥≈Ι≤’ώ«βΤΉΆΦœ‘ Ψ”–»ΐ÷ΊΖεΘ§ΖεΟφΜΐ÷°±»ΈΣ3ΘΚ2ΘΚ3Θ§ΗΟ”–ΜζΈο≤ΜΚ§”–CH3Θ≠OΘ≠Θ§‘ρAΒΡΫαΙΙΦρ ΫΈΣ
_______________ΓΘ
Θ®2Θ©–¥≥ω Β―ι “÷Τ±ΗAΒΡΜ·―ßΖΫ≥Χ Ϋ_________________________________________ΓΘ
ΔρΘ°±ΫΦΉΥαΦΉθΞ( ) «≥Θ”ΟœψΨΪΘ§ΙψΖΚ”Ο”Ύ ≥ΤΖΓΔΜ·Ή±ΤΖΒ»––“ΒΘ§Ω…¥”Ή‘»ΜΫγ÷–Χα»ΓΘ§“≤Ω…»ΥΙΛΚœ≥…ΓΘ Β―ι “œ÷“‘ ≥ΤΖΖάΗ·ΦΝ[÷ς“Σ≥…Ζ÷ΈΣ±ΫΦΉΥαΡΤ(
) «≥Θ”ΟœψΨΪΘ§ΙψΖΚ”Ο”Ύ ≥ΤΖΓΔΜ·Ή±ΤΖΒ»––“ΒΘ§Ω…¥”Ή‘»ΜΫγ÷–Χα»ΓΘ§“≤Ω…»ΥΙΛΚœ≥…ΓΘ Β―ι “œ÷“‘ ≥ΤΖΖάΗ·ΦΝ[÷ς“Σ≥…Ζ÷ΈΣ±ΫΦΉΥαΡΤ( )]ΓΔΦΉ¥ΦΈΣ‘≠Νœ÷Τ±Η±ΫΦΉΥαΦΉθΞΓΘ“―÷ΣΘΚ
)]ΓΔΦΉ¥ΦΈΣ‘≠Νœ÷Τ±Η±ΫΦΉΥαΦΉθΞΓΘ“―÷ΣΘΚ
| | »έΒψ Γφ | Ζ–Βψ Γφ | Υ°»ή–‘ |
| ΦΉ¥Φ | ΓΣ97.8 | 64.7 | “Ή»ή |
| ±ΫΦΉΥαΘ®“Μ‘Σ»θΥαΘ© | 122.4 | 249.3 | ≥ΘΈ¬ΘΚ0.17gΘΜ100ΓφΘΚ6.8g |
| ±ΫΦΉΥαΦΉθΞ | ΓΣ12.3 | 198 | Ρ―»ή |
Κœ≥…±ΫΦΉΥαΦΉθΞΒΡΝς≥Χ»γœ¬ΘΚ

«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©Έ¬Ε»ΔΌ‘ΦΈΣ_________ΓφΘ§≤ΌΉςΔέΈΣ_________Θ§≤ΌΉςΔήΈΣ__________ΓΘ
Θ®2Θ©ΒΎΔΎ≤ΫΦ”»»ΒΡΡΩΒΡ «______________________________________ΓΘ
Θ®3Θ©―Γ‘ώΚœ ΒΡ÷Τ±Η±ΫΦΉΥαΦΉθΞΒΡΉΑ÷ΟΘΚ______________ΓΘ




A B C D
Θ®4Θ©±ΫΦΉΥαΦΉθΞ”–Εύ÷÷Ά§Ζ÷“λΙΙΧεΘ§–¥≥ωΖϊΚœœ¬Ν–ΧθΦΰΒΡ»Έ“β“Μ÷÷Ά§Ζ÷“λΙΙΧεΒΡΫαΙΙΦρ Ϋ_______ΓΘΔΌΈΣΖΦœψΜ·ΚœΈο ΔΎΚ§”–»©Μυ ΔέΡή”κΫπ τNaΖ¥”Π
Ή Νœœ‘ ΨΘΚΟΨ”κ±ΞΚΆΧΦΥα«βΡΤ»ή“ΚΖ¥”Π≤ζ…ζ¥σΝΩΤχΧεΚΆΑΉ…Ϊ≤Μ»ήΈοΓΘΡ≥Ά§―ßΆ®Ιΐ»γœ¬ Β―ιΧΫΨΩΖ¥”Π‘≠άμ≤Δ―ι÷Λ≤ζΈοΓΘ
Β―ιIΘΚ”Ο…Α÷Ϋ≤Ν»ΞΟΨΧθ±μΟφ―θΜ·ΡΛΘ§ΫΪΤδΖ≈»κ Δ ΝΩΒΈ”–Ζ”ΧΣΒΡ±ΞΚΆΧΦΥα«βΡΤ»ή“ΚΒΡ…’±≠÷–Θ§―ΗΥΌΖ¥”ΠΘ§≤ζ…ζ¥σΝΩΤχ≈ίΚΆΑΉ…Ϊ≤Μ»ήΈοΘ§»ή“ΚΒΡ«≥Κλ…ΪΦ”…νΓΘ
Θ®1Θ©ΗΟΆ§―ßΕ‘Ζ¥”Π÷–≤ζ…ζΒΡΑΉ…Ϊ≤Μ»ήΈοΉω≥ω»γœ¬≤¬≤βΘΚ
≤¬≤β1ΘΚΑΉ…Ϊ≤Μ»ήΈοΩ…ΡήΈΣΓΓΓΓΓΓΓΓΓΓ
≤¬≤β2ΘΚΑΉ…Ϊ≤Μ»ήΈοΩ…ΡήΈΣMgCO3
≤¬≤β3ΘΚΑΉ…Ϊ≤Μ»ήΈοΩ…ΡήΈΣΦν ΫΧΦΥαΟΨ[yMg(OH)2?xMgCO3]
Θ®2Θ©ΈΣΝΥ»ΖΕ®≤ζΈοΘ§Ϋχ––“‘œ¬Ε®–‘ Β―ιΘΚ
| Β―ι–ρΚ≈ | Β ―ι | Β―ιœ÷œσ | Ϋα ¬έ |
| Β―ιΔρ | ΫΪ Β―ιI÷– ’Φ·ΒΫΒΡΤχΧεΒψ»Φ | Α≤Ψ≤»Φ…’Θ§ Μπ―φ≥ Β≠άΕ…Ϊ | ΤχΧε≥…Ζ÷ΈΣ ΓΓ ΔΌ |
| Β―ιΔσ | ΫΪ Β―ιI÷–ΒΡΑΉ…Ϊ≤Μ»ήΈο¬Υ≥ωΓΔœ¥Β”Θ§»Γ…ΌΝΩΦ”»κΉψΝΩ ΔΎ | Δέ | ΑΉ…Ϊ≤Μ»ήΈο÷–Κ§”–MgCO3 |
| Β―ιΔτ | »Γ Β―ιΔσ÷–ΒΡ¬Υ“ΚΘ§œρΤδ÷–Φ”»κ ΝΩΓΓ ΔήΓΓ œΓ»ή“Κ | ≤ζ…ζΑΉ…Ϊ≥ΝΒμΘ§»ή“ΚΚλ…Ϊ±δ«≥ | »ή“Κ÷–¥φ‘ΎCO32- άκΉ” |
Β―ιΔσ÷–œ¥Β”ΒΡ≤ΌΉςΖΫΖ® « ΓΘ
Θ®3Θ©ΈΣΫχ“Μ≤Ϋ»ΖΕ® Β―ιIΒΡΑΉ…Ϊ≤Μ»ήΈοΒΡ≥…Ζ÷Θ§Ϋχ––“‘œ¬Ε®ΝΩ Β―ιΘ§ΉΑ÷Ο»γΆΦΥυ ΨΘΚ

≥Τ»ΓΗ…‘οΓΔ¥ΩΨΜΒΡΑΉ…Ϊ≤Μ»ήΈο 4.52 gΘ§≥δΖ÷Φ”»»÷Ν≤Μ‘Ό≤ζ…ζΤχΧεΈΣ÷ΙΘ§≤Δ ΙΖ÷Ϋβ≤ζ…ζΒΡΤχΧε»Ϊ≤ΩΫχ»κΉΑ÷ΟAΚΆB÷–ΓΘ Β―ιΚσΉΑ÷ΟA‘ω÷Ί0.36 gΘ§ΉΑ÷ΟB‘ω÷Ί1.76 gΓΘ
ΉΑ÷ΟCΒΡΉς”Ο « ΘΜ
ΑΉ…Ϊ≤Μ»ήΈοΒΡΜ·―ß ΫΈΣΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓ ΓΘ
Θ®4Θ©–¥≥ωΟΨ”κ±ΞΚΆΧΦΥα«βΡΤ»ή“ΚΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ ΓΘ
ΫΪ“ΜΕ®ΝΩΧζΝΘΖ≈»κΡ≥≈®Ε»œθΥα÷–Θ§≥δΖ÷Ζ¥”ΠΚσΒΟΒΫ»ή“ΚX≤Δ ’Φ·ΒΫΤχΧεYΓΘ
Θ®1Θ©ΈΣΧΫΨΩ»ή“ΚX÷–Χζ‘ΣΥΊΒΡΦέΧ§Θ§…ηΦΤ»γœ¬ Β―ιΘΚ
“©ΤΖΚΆ“«ΤςΘΚ0Θ°1molΓΛL-1KSCN»ή“ΚΓΔ0Θ°1molΓΛL-1KI»ή“ΚΓΔ0Θ°2molΓΛL-1Υα–‘ΗΏΟΧΥαΦΊ»ή“ΚΓΔ¬»Υ°ΓΔ ‘ΙήΚΆΒΈΙήΓΘ
«κΗυΨί Β―ι…ηΦΤΘ§Χν–¥œ¬Ν– Β―ι±®ΗφΘΚ
| Β―ι≤Ϋ÷η | Β―ι≤ΌΉς | œ÷œσ”κΫα¬έ | άκΉ”ΖΫ≥Χ Ϋ |
| ΒΎ1≤Ϋ | »Γ2~3mL»ή“ΚΉΑ”Ύ ‘ΙήΘ§œρ ‘Ιή÷–ΒΈΦ”ΦΗΒΈKSCN»ή“Κ | | |
| ΒΎ2≤Ϋ | | »τ»ή“ΚΉœ…ΪΆ »ΞΘ§‘ρ»ή“Κ Κ§”–Fe2+ΘΜ»τΈόΟςœ‘±δΜ·Θ§ ‘ρ≤ΜΚ§Fe2+ | |
Ρ≥Μ·―ß–ΓΉι≤…”ΟάύΥΤ÷Τ““Υα““θΞΒΡΉΑ÷ΟΘ®»γΆΦΘ©Θ§“‘ΜΖΦΚ¥Φ÷Τ±ΗΜΖΦΚœ©ΘΚ
“―÷ΣΘΚ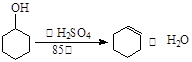
| | ΟήΕ» Θ®g/cm3Θ© | »έΒψ Θ®ΓφΘ© | Ζ–Βψ Θ®ΓφΘ© | »ήΫβ–‘ |
| ΜΖΦΚ¥Φ | 0.96 | 25 | 161 | Ρή»ή”ΎΥ° |
| ΜΖΦΚœ© | 0.81 | Θ≠103 | 83 | Ρ―»ή”ΎΥ° |
ΫΪ12.5mLΜΖΦΚ¥ΦΦ”»κ ‘ΙήA÷–Θ§‘ΌΦ”»κ1mL≈®ΝρΥαΘ§“Γ‘»ΚσΖ≈»κΥι¥…Τ§Θ§ΜΚ¬ΐΦ”»»÷ΝΖ¥”ΠΆξ»ΪΘ§‘Ύ ‘ΙήCΡΎΒΟΒΫΜΖΦΚœ©¥÷ΤΖΓΘ
ΔΌA÷–Υι¥…Τ§ΒΡΉς”Ο « Θ§ΒΦΙήB≥ΐΝΥΒΦΤχΆβΜΙΨΏ”–ΒΡΉς”Ο « ΓΘ
ΔΎ ‘ΙήC÷Ο”Ύ±υΥ°‘Γ÷–ΒΡΡΩΒΡ « ΓΘ
Θ®2Θ©÷Τ±ΗΨΪΤΖ
ΔΌΜΖΦΚœ©¥÷ΤΖ÷–Κ§”–ΜΖΦΚ¥ΦΚΆ…ΌΝΩΥα–‘‘”÷ Β»ΓΘΦ”»κ±ΞΚΆ ≥―ΈΥ°Θ§’ώΒ¥ΓΔΨ≤÷ΟΓΔΖ÷≤ψΘ§ΜΖΦΚœ©‘Ύ ≤ψΘ®ΧνΓΑ…œΓ±ΜρΓΑœ¬Γ±Θ©Θ§Ζ÷“ΚΚσ”Ο Θ®Χν»κ±ύΚ≈Θ©œ¥Β”ΓΘ
AΘ°KMnO4»ή“Κ BΘ°œΓH2SO4 CΘ°Na2CO3»ή“Κ
ΔΎ‘ΌΫΪΜΖΦΚœ©Α¥ΆΦΉΑ÷Ο’τΝσΘ§ά以հ¥” ΩΎΫχ»κΓΘ’τΝσ ±“ΣΦ”»κ…ζ ·Μ“Θ§ΡΩΒΡ «: ΓΘ
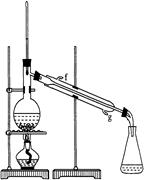
Δέ ’Φ·≤ζΤΖ ±Θ§ΩΊ÷ΤΒΡΈ¬Ε»”Π‘Ύ Ήσ”“Θ§ Β―ι÷ΤΒΟΒΡΜΖΦΚœ©ΨΪΤΖ÷ ΝΩΒΆ”Ύάμ¬έ≤ζΝΩΘ§Ω…ΡήΒΡ‘≠“ρ « ΘΏΘΏΘΏΘΏΘΏΘΏΘΏ
AΘ°’τΝσ ±¥”70ΓφΩΣ Φ ’Φ·≤ζΤΖ BΘ°ΜΖΦΚ¥Φ ΒΦ ”ΟΝΩΕύΝΥ
CΘ°÷Τ±Η¥÷ΤΖ ±ΜΖΦΚ¥ΦΥφ≤ζΤΖ“ΜΤπ’τ≥ω
Θ®3Θ©“‘œ¬«χΖ÷ΜΖΦΚœ©ΨΪΤΖΚΆ¥÷ΤΖΒΡΖΫΖ®Θ§ΚœάμΒΡ «ΘΏΘΏΘΏΘΏΘΏΘΏΘΏ
AΘ°”ΟΥα–‘ΗΏΟΧΥαΦΊ»ή“Κ BΘ°”ΟΫπ τΡΤ CΘ°≤βΕ®Ζ–Βψ
Θ®4Θ©”…ΜΖΦΚœ©‘ΌΉΣΜ·ΈΣΜΖΦΚ¥ΦΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚΘΏΘΏΘΏΘΏΘΏΘΏΘΏΘΏΘΏΘΏΘΏΘΏΘΏΘΏ
ΈΣΧΫΨΩCl2ΓΔΤ·ΑΉΖέΒΡ÷Τ±ΗΦΑ”–ΙΊ–‘÷ Θ§Ρ≥–Υ»Λ–ΓΉι…ηΦΤ≤ΔΫχ––ΝΥ“‘œ¬ Β―ιΧΫΨΩΓΘ«κΜΊ¥π“‘œ¬Έ ΧβΘΚ
Θ®1Θ© Β―ι “Ρβ”Οœ¬Ν–ΉΑ÷Ο÷Τ±ΗΗ…‘ο¥ΩΨΜΒΡ¬»ΤχΘ§«κΑ¥’’ΤχΧε¥”Ήσœρ”“ΝςΕ·ΒΡΖΫœρΫΪ“«ΤςΫχ––Ν§Ϋ”ΘΚHΓζ_______ΓΔ_______Γζ_______ΓΔ_______Γζ_______ΘΜΤδ÷–ΙψΩΎΤΩΔρ÷–ΒΡ ‘ΦΝΈΣ_______ΓΘ

Θ®2Θ©–¥≥ωΙΛ“Β…œ”Ο¬»ΤχΚΆ ·Μ“»ι÷Τ»ΓΤ·ΑΉΖέΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ Ϋ_______ΘΜ
Θ®3Θ© Β―ι “”–“ΜΤΩΟήΖβ≤Μ―œΒΡΤ·ΑΉΖέ―υΤΖΘ§Τδ÷–ΩœΕ®¥φ‘ΎCaCl2ΓΘ«κ…ηΦΤ Β―ιΘ§ΧΫΨΩΗΟ―υΤΖ÷–≥ΐCaCl2ΆβΜΙΚ§”–ΒΡΤδΥϊΙΧΧεΈο÷ ΓΘ
ΔΌΧα≥ωΚœάμΦΌ…ηΓΘ
ΦΌ…η1ΘΚΗΟΤ·ΑΉΖέΈ¥±δ÷ Θ§ΜΙΚ§”–Ca(ClO)2
ΦΌ…η2ΘΚΗΟΤ·ΑΉΖέ»Ϊ≤Ω±δ÷ Θ§ΜΙΚ§”–______ΘΜ
ΦΌ…η3ΘΚΗΟΤ·ΑΉΖέ≤ΩΖ÷±δ÷ Θ§ΜΙΚ§”–Ca(ClO)2ΚΆCaCO3ΓΘ
ΔΎ…ηΦΤ Β―ιΖΫΑΗΘ§Ϋχ–– Β―ιΓΘ«κ‘Ύœ¬±μ÷––¥≥ω Β―ι≤Ϋ÷ηΓΔ‘ΛΤΎœ÷œσΚΆΫα¬έΓΘ
œό―Γ”ΟΒΡ“«ΤςΚΆ“©ΤΖΘΚ ‘ΙήΓΔΒΈΙήΓΔ¥χΒΦΙήΒΡΒΞΩΉ»ϊΓΔ’τΝσΥ°ΓΔΉ‘ά¥Υ°ΓΔΤΖΚλ»ή“ΚΓΔ1 molΓΛL-1 HCl»ή“ΚΓΔ–¬÷Τ≥Έ«ε ·Μ“Υ°ΓΘΘ®Χα ΨΘΚ≤Μ±ΊΦλ―ιCa2+ΚΆCl-ΓΘΘ©
| | Β―ι≤Ϋ÷η | ‘ΛΤΎœ÷œσ”κΫα¬έ |
| ≤Ϋ÷η1 | »Γ…ΌΝΩ…œ ωΤ·ΑΉΖέ”Ύ ‘Ιή÷–Θ§œ»Φ”»κ »ήΫβΚσΘ§‘ΌΑ―…ζ≥…ΒΡΤχΧεΆ®»κ ΓΘ | »τ Θ§‘ρΦΌ…η1≥…ΝΔΘΜ »τ Θ§‘ρΦΌ…η2ΜρΦΌ…η3≥…ΝΔΓΘ |
| ≤Ϋ÷η2 | “―»ΖΕ®Τ·ΑΉΖέ±δ÷ Θ§‘ρΝμ»Γ…ΌΝΩ…œ ωΤ·ΑΉΖέ”Ύ ‘Ιή÷–Θ§œ»Φ”»κ ΝΩ1 molΓΛL-1 HCl»ή“ΚΘ§‘ΌΦ”»κ ΓΘ | »τ Θ§‘ρΦΌ…η2≥…ΝΔΘΜ »τ Θ§‘ρΦΌ…η3≥…ΝΔΓΘ |
Ρ≥ Β―ι–ΓΉιΕ‘Τ’Ά®–ΩΟΧΖœΗ…Βγ≥ΊΡΎΒΡΚΎ…ΪΙΧΧεΫχ––ΧΫΨΩΘ§…ηΦΤ»γœ¬ΖΫΑΗΘΚ
ΦΚ÷ΣΘΚIΓΔΤ’Ά®–ΩΟΧΒγ≥ΊΒΡΚΎ…ΪΈο÷ ÷ς“Σ≥…Ζ÷ΈΣMnO2ΓΔNH4ClΓΔZnCl2Β»Έο÷ ΓΘ
IIΓΔ«β―θΜ·–ΩΈΣΑΉ…ΪΖέΡ©Θ§≤Μ»ή”ΎΥ°Θ§»ή”ΎΥαΓΔ«ΩΦν»ή“ΚΚΆΑ±Υ°ΓΘ
«κΜΊ¥π“‘œ¬Έ ΧβΘΚ
Θ®1Θ©ΔΎ≤ΌΉςΒΡΟϊ≥Τ «___________ΓΘ
Θ®2Θ©Ρ≥Ά§―ß≤¬œκ»ή“ΚAΒΡ≥…Ζ÷Κ§”–NH4ClΚΆZnCl2Θ§«κΡψ…ηΦΤ“ΜΗω Β―ιΖΫΑΗΘ§―ι÷ΛΤδ≤¬œκ’ΐ»ΖΘ§“Σ«σ‘Ύ¥πΧβΩ®…œΑ¥œ¬±μΗώ Ϋ–¥≥ω Β―ι≤ΌΉςΓΔ‘ΛΤΎœ÷œσΚΆΫα¬έΓΘ
œό―Γ ‘ΦΝΘΚ’τΝσΥ°ΓΔ2moLΓΛLΘ≠1 HCI ΓΔ2 moLΓΛLΘ≠1 HNO3 ΓΔ2 moLΓΛLΘ≠1 NH3ΓΛH2OΓΔ6 moLΓΛLΘ≠1 NaOHΓΔ0.1 moLΓΛLΘ≠1 KSCNΓΔ0.1 moLΓΛLΘ≠1 BaCl2ΓΔ0.1 moLΓΛLΘ≠1 AgNO3ΓΔΉœ…Ϊ ·»ο ‘“ΚΓΔΚλ…Ϊ ·»ο ‘÷Ϋ
| Β―ι≤ΌΉς | ‘ΛΤΎœ÷œσ | Ϋα¬έ |
| ≤Ϋ÷η1ΘΚΗς»Γ…ΌΝΩ»ή“ΚAΖ÷ΉΑaΓΔbΓΔc»ΐ÷ß ‘ΙήΘ§Άυa ‘ΙήΘ§__ __________________________ | ”–ΑΉ…Ϊ≥ΝΒμ≤ζ…ζ | ΥΒΟς»ή“ΚAΚ§”–ClΘ≠ |
| ≤Ϋ÷η2ΘΚΆυb ‘ΙήΘ§__________ __________________________ | ______________________ | _______________________ |
| ≤Ϋ÷η3ΘΚΆυc ‘ΙήΘ§__________ __________________________ | œ»≤ζ…ζ_______________, Κσ____________________ | ΥΒΟς»ή“ΚAΚ§”–Zn2+ |
Θ®3Θ©»Γ…ΌΝΩΙΧΧεcΖ≈»κ ‘ΙήΘ§ΒΈΦ”»κΥΪ―θΥ°Θ§Ιέ≤λΒΫ”–ΤχΧε≤ζ…ζΘ§–¥≥ωΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ_______________ΓΘ
Θ®4Θ©ΈΣ≤βΕ®ΖœΗ…Βγ≥Ί÷–Εΰ―θΜ·ΟΧΒΡ÷ ΝΩΖ÷ ΐΘ§Ϋχ––œ¬Οφ Β―ιΘΚΉΦ»Ζ≥Τ»ΓagΖœ«ßΒγ≥ΊΙΧΧεΘ§»ή”ΎœΓΝρΥαΘ§Φ”»κΒβΜ·ΦΊ»ή“ΚΘ§≥δΖ÷Ζ¥”ΠΚσΘ§”Οbmol/LΝρ¥ζΝρΥαΡΤ±ξΉΦ»ή“ΚΒΈΕ®Θ§”ΟΒμΖέΉς÷Η ΨΦΝΘ§ΒΈΕ®÷Ν÷’ΒψΘ§÷ΊΗ¥ Β―ιΘ§ΤΫΨυœϊΚΡΝρ¥ζΝρΥαΡΤ±ξΉΦ»ή“ΚΒΡΧεΜΐΈΣvmLΘ§‘ρΖœΒγ≥Ί÷–Εΰ―θΜ·ΟΧΒΡ÷ ΝΩΖ÷ ΐΒΡΦΤΥψ±μ¥ο ΫΈΣΘΚ________________________________ΓΘ
Θ®ΒΈΕ®”–ΙΊΖ¥”ΠΘΚMnO2+2IΘ≠+4H+=Mn2++I2+2H2OΘΜI2+2S2O32Θ≠=2IΘ≠+S4O62Θ≠Θ©

