��Ŀ����
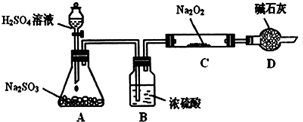
ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ��
��֪��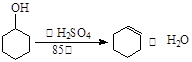
| | �ܶ� ��g/cm3�� | �۵� ���棩 | �е� ���棩 | �ܽ��� |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | ��103 | 83 | ������ˮ |
��12.5mL�����������Թ�A�У��ټ���1mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ�������� ������B���˵�������е������� ��
���Թ�C���ڱ�ˮԡ�е�Ŀ���� ��
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ�� �㣨��ϡ����¡�������Һ���� �������ţ�ϴ�ӡ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
���ٽ�����ϩ��ͼװ��������ȴˮ�� �ڽ��롣����ʱҪ������ʯ�ң�Ŀ����: ��
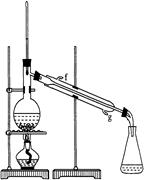
���ռ���Ʒʱ�����Ƶ��¶�Ӧ�� ���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ���� �ߣߣߣߣߣߣ�
A������ʱ��70�濪ʼ�ռ���Ʒ B��������ʵ����������
C���Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ������������ǣߣߣߣߣߣߣ�
A�������Ը��������Һ B���ý����� C���ⶨ�е�
��4���ɻ���ϩ��ת��Ϊ�������Ļ�ѧ����ʽΪ���ߣߣߣߣߣߣߣߣߣߣߣߣߣ�
(1) �ٷ�ֹ���У� ������ �ڷ�ֹ����ϩ�Ļӷ���
(2) ���ϣ� C�� ��g����ȥˮ�֡� �� 83�� �� C��
��3��B��C��
��4������ϩ + H2O����������
���������������1���ټ���Һ��ʱ��Ϊ�˷�ֹҺ�屬�У������ü����ʯ�����Ƭ���ӷ������ʷ�ӦʱΪ����������ʣ�һ��Ҫ����������װ�ã�����B�������������ã��ڷ�Ӧ���ɵIJ���е�ϸߣ��ӷ���Ӧ���ñ�ˮ��ȴ����ֹ����ϩ�Ļӷ�����2���������ܶȱ�ˮС����ˮ���ʱ������ϩ���ϲ㣻Ҫ��ȥ��Ʒ�е������ʣ�����̼���Ƶȼ�����Һ��ȥ����ʵ����һ�����������ȴ������ʱ������ʯ��Ŀ������ˮ��Ӧ����ֹ���Ʒһ������������ռ���Ʒʱ�����Ƶ��¶�Ӧ�û���ϩ�ӷ���������2��������������Һ�У������¶ȿɿ�����83�桪��161�棬����Ϊ��ˮԡ���Ⱥ����������ĵȷ���ɿ����¶ȵ�һЩ 83�漴�ɣ�ʵ���ƵõĻ���ϩ��Ʒ�����������۲�����˵������ϩ������ʧ�ˣ��ʴ�ΪC����3����Ʒ�к��л����������Ʒ�Ӧ���һ����е㲻�̶����뻷��ϩ�е㲻ͬ��
���㣺���������Ʊ�ʵ���в�����Ŀ�ġ�ԭ�������������й����⡣

ijУ��ѧ��ȤС��Ϊ��̽����������������������ͭ���ʣ���ijŨ�����ᷴӦ�����������̽�����
̽��һ
��1����������Ͷ��ijŨ�������У�ijͬѧ�۲��ʵ������ʱ���֣���Ӧ����һ��ʱ���Ӧ��ʼ�ӿ졣�������Ӧ�ӿ�Ŀ���ԭ���_____________��_____________��
��2������ȡ������10 g����ijŨ�������У���ַ�Ӧ��õ���ҺX���ռ�������Y��Ϊ��̽����ҺX����Ԫ�صļ�̬����ͬѧ�������ʵ�飺
��ҩƷ��������0.1mol/L KSCN��Һ��0.1mol/L KI��Һ��0.2mol/L���Ը��������Һ����ˮ�ȣ��Թܺ͵ιܡ�
������Ƽ�ʵ�飬̽�����������Ƿ���ȷ����д����ʵ�鱨�棺
| ʵ�鲽�� | ���� | ���� | ���ӷ���ʽ |
| ��һ�� | ȡ2��3 mL��Һװ���Թܣ����Թ��м��뼸��KSCN��Һ�� | | |
| �ڶ��� | | ����Һ��ɫ��ȥ������Һ����Fe2+���������Ա仯����Fe3+�� | |
Ϊ��̽������Y�ijɷ֣�ͬѧ�����������װ�ã�������NO2ת��ΪN2O4��

��3��װ���ҵ����ã� ��
��4��װ�ñ��ռ��������ͨ�����ݹ���������к���ɫ�������ɣ��ܷ�ȷ������Y�к�NO��˵������__________ __��
��5��ͬѧ��Ϊ��̽������Y����ɣ��ڱ�״���½�224mL����Yͨ��������NaOH��Һ�У����屻��ȫ���գ�������Һ����0.1500mol/L����KMnO4��Һ�ζ�������20.00mLKMnO4��Һ��������Y��NO��NO2�������Ϊ ������֪2NO2+2NaOH��NaNO3+NaNO2+H2O��NO2+NO+2NaOH��2NaNO2+H2O��
ij��Ͻ�����ĩ,��Mg��,������Al��Zn�е�һ�ֻ�����,��������10%����.ij�о�С�����ʵ��̽���û�Ͻ�����ĩ������пԪ�صĴ���.
�����Լ�����Ʒ��pH��ֽ��ϡH2SO4��NaOH��Һ��ϡNH3��H2O.
��С��̽���������£�
��������ϣ�
| ��þ������п��������ɫ�Ľ���; ��п(Zn)������NaOH��Һ��Ӧ����H2; ��Zn(OH)2Ϊ��ɫ����,������ˮ,������ǿ�NH3��H2O; ��Zn2+���γ���������Zn(NH3)4��2+,���������ǿ��ֽ�����Zn2+��NH4+. |
��1������٣��û�Ͻ�����ĩ�г�þ�����________Ԫ��
����ڣ��û�Ͻ�����ĩ�г�þ�����________Ԫ��
����ۣ��û�Ͻ�����ĩ�г�þ���������пԪ��
��ʵ��̽����
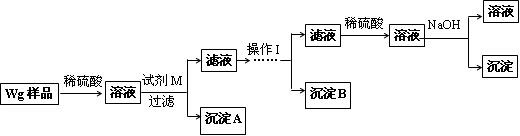
��ͬѧ���ڼ�������ʵ�鷽�����£�
��ͬѧͬ�����ڼ���3�����һʵ�鷽�����£�
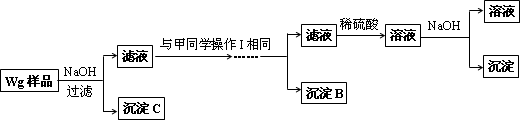
��2���Լ�M��____________,����B��_____________.
��2���Լ�M��____________,����B��_____________.
��3����ͬѧ��Ϊ��ͬѧ�ķ����ȼ�ͬѧ�ĺ�,������_______________________.
��4����ͬѧ�о��˼ס�����ͬѧ�ķ�����,������һ�ַ����Ļ����������㷽���ⶨ��Wg��Ʒ�н���þ(Mg)������,���ķ�����____________________________.
��5�����������Ҫ�����ǣ�����Һ����μ���_______________,ֱ�����ɵij����պ��ܽ⣬�ټ���������____________________.
ʵ������ȡ��ϩ�ķ�Ӧԭ��Ϊ��CH3CH2OH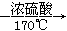 CH2=CH2����H2O����Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������
CH2=CH2����H2O����Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������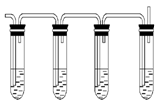
�Իش��������⣺
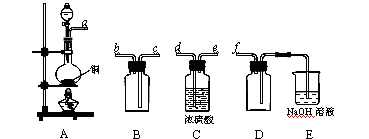
��1��ͼ�Т٢ڢۢ�װ��ʢ�ŵ��Լ��ֱ��ǣ���ѡ����ĸ������_________����_________��
| A��Ʒ����Һ | B��NaOH��Һ | C��Ũ���� | D�����Ը��������Һ |
��3��ȷ֤��ϩ���ڵ�������_______________________________________________________________��
ʵ������ȡ���ᶡ����ʵ��װ�������¼ס�������װ�ÿɹ�ѡ�á�

���ף� ���ң�
�Ʊ����ᶡ�����漰���й����ʵ��������ʼ��±�
| | ���� | 1������ | ���ᶡ�� |
| �۵�(��) | 16��6 | ��89��5 | ��73��5 |
| �е�(��) | 117��9 | 117 | 126��3 |
| �ܶ�(g/cm3) | 1��05 | 0��81 | 0��88 |
| ˮ���� | ���� | ���� (9g/100gˮ) | �� |
��1����ȡ���ᶡ����װ��Ӧѡ��___________(��ס����ҡ�)����ѡ��һ��װ�õ�������______________________________________________________________��
��2����ʵ���������г������������ᶡ���⣬���������ɵ��л��������У�д���ṹ��ʽ��________________________________________________________________��
��3��������Ӧ��һ�����淴Ӧ��Ϊ���1�������������ʣ�д�����ֿ��еķ�����
�� ����
��4�����Ʊ����ᶡ�����õĻ�����з��롢�ᴿ���ᶡ��ʱ����Ҫ�����ಽ����������ͼʾ�IJ����У��϶���Ҫ�Ļ�ѧ������________________��ѡ��𰸱�ţ���





A B C D
��5���л���ķ�������У�������Ҫʹ�÷�Һ©����������ʹ�÷�Һ©��ǰ����_______________��ijͬѧ�ڽ��з�Һ����ʱ��������Һ�����������������ԭ�����Һ©�����������⣬���� ��
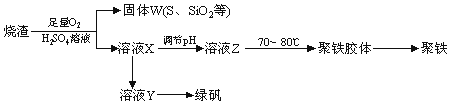
�ִ�п��Ʒ�ӹ���ҵ���յķ���������ZnO��FeO��Fe2O3��CuO��Al2O3�����ʣ�����ȡ����п���������£�
�й�����������ȫ������pH���±���
| ������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 | Zn(OH)2 |
| pH | 5.2 | 3.2 | 9.7 | 6.7 | 8.0 |
��l������������У�Ҫ���пԪ�صĽ����ʣ����Բ�ȡ ��ʩ��
��2�����������жദ�漰�����ˡ���ʵ�����й��˲�����Ҫʹ�õIJ��������� ��
��3���ڡ�����I�������У�����Һ����pH=4��Ŀ���� ���ڡ�����II������Һ��pHԼΪ6����˲�����ʱ�����к��� ��
��4���ڡ�̼���ϳɡ��У����ɵIJ���֮һΪ��ʽ̼��п[Zn2��OH��2CO3]��ͬʱ�ų�CO2����д���÷�Ӧ�Ļ�ѧ����ʽ ��
��5������Һ����ȡNaNO3����IJ�������Ϊ ��
��6����ʵ�������ϴ�ӹ��˳��ļ�ʽ̼��п�� ��