��Ŀ����
12����ѧ��Ӧԭ�������о����ʵı仯�����ش���1����ij�ܷ������г�������ʵ�������A��B��-���¶��£��������·�Ӧ��
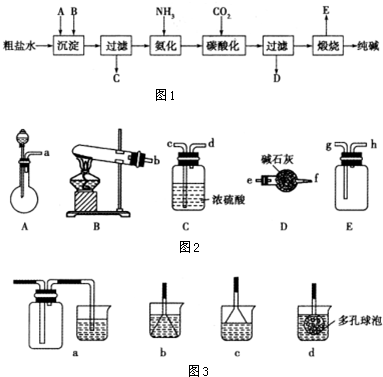
aA��g��+bB��g��?2C��g������ƽ���ֻ�ı�һ����Ӧ���������������������ʵ����ʵ���Ũ�ȡ���ѧ��Ӧ������ʱ��仯��ϵ���߷ֱ���ͼ��ʾ��
��7min��A�Ļ�ѧ��Ӧ����v��A��=0.08mol•L-1•min-1��
��b=1��
�۷�Ӧ�ġ�H��O�����������=����������40min�ı�ķ�Ӧ�����������¶ȣ�
��20-30min��30〜40min�Ļ�ѧƽ�ⳣ����ϵ�ǣ�ǰ�ߵ��ں��ߣ�����ڡ��������ڡ���С�ڡ�����
��2��25��ʱ����0.2mol•L-1��ij��HX��μ��뵽25mL0.1mol•L-1NaOH��Һ�У�ǡ����ȫ��Ӧʱ��������Һ��pH��7��
�ٵ��μӵ����С��12.5mLʱ������HX�ĵ��룬ˮ�ĵ���̶Ȼ�����������������ޱ仯������С������
�����μ���15mLʱ��Һ�����ԣ���HA�ĵ��볣��K=5��10-7mol•L-1��
�۵��μ���25mLʱ����Һ������Ũ���ɴ�С��˳����c��X-����c��Na+����c��H+����c��OH-����
���� ��1����7min��A�Ļ�ѧ��Ӧ����v��A��=$\frac{��c}{��t}$��
�����ʵ����仯��֮�ȵ���ϵ��֮�ȣ����������
��40minʱ�����淴Ӧ���ʶ��������淴Ӧ���ʴ�������Ӧ���ʣ�ƽ�������ƶ���Ӧ�������¶ȣ�������ӦΪ���ȷ�Ӧ��
��30��40 minֻ�з�Ӧ���ʽ����ˣ���Ӧ�����������Ũ��˲ʱ���ͣ���Ӧ�Դ���ƽ��״̬���ʲ������¶ȱ仯�����ǽ�����ѹǿ����ѧƽ�ⳣ�����¶ȵĺ�����
��2���ٵ��μӵ����С��12.5mLʱ������HX�ĵ��룬��Һ�����������ӵ�Ũ����С������ˮ�ĵ���̶�������
��K=$\frac{c��{H}^{+}��•c��{A}^{-}��}{c��HA��}$������c��H+��=10-7mol/L��c��Na+��=c��A-��=$\frac{25��0.1}{15+25}$=0.0625mol/L������c��HA��=$\frac{0.2��15}{15+25}$-$\frac{25��0.1}{15+25}$=0.0125mol/L��������⣻
�۵��μ���25mLʱ���õ���������Ũ�ȵ�HX��NaX�Ļ����Һ����HX�ĵ���Ϊ������Һ�����ԣ��Դ�ȷ������Ũ�ȵĴ�С��
��� �⣺��1����7min��A�Ļ�ѧ��Ӧ����v��A��=$\frac{��c}{��t}$=$\frac{2-1.44}{7}$=0.08mol•L-1•min-1���ʴ�Ϊ��0.08mol•L-1•min-1��
����Ϊ0��20min��A��Ũ�ȱ仯��Ϊ2-1=1mol•L-1����C��Ũ�ȱ仯��Ϊ2-0=2mol•L-1������$\frac{a}{2}=\frac{1}{2}$����a=1������Ϊ��30minʱֻ�з�Ӧ���ʽ����ˣ���Ӧ�����������Ũ��˲ʱ���ͣ���Ӧ�Դ���ƽ��״̬���ʲ������¶ȱ仯�����ǽ�����ѹǿ��˵�����ߵļ�������ȣ�����a+b=2����b=1���ʴ�Ϊ��1��
��40minʱ�����淴Ӧ���ʶ��������淴Ӧ���ʴ�������Ӧ���ʣ�ƽ�������ƶ���Ӧ�������¶ȣ�������ӦΪ���ȷ�Ӧ���ʴ�Ϊ�����������¶ȣ�
��30��40 minֻ�з�Ӧ���ʽ����ˣ���Ӧ�����������Ũ��˲ʱ���ͣ���Ӧ�Դ���ƽ��״̬���ʲ������¶ȱ仯�����ǽ�����ѹǿ����ѧƽ�ⳣ�����¶ȵĺ������ʴ�Ϊ�����ڣ�
��2���ٵ��μӵ����С��12.5mLʱ������HX�ĵ��룬��Һ�����������ӵ�Ũ����С������ˮ�ĵ���̶������ʴ�Ϊ��������
��K=$\frac{c��{H}^{+}��•c��{A}^{-}��}{c��HA��}$������c��H+��=10-7mol/L��c��Na+��=c��A-��=$\frac{25��0.1}{15+25}$=0.0625mol/L������c��HA��=$\frac{0.2��15}{15+25}$-$\frac{25��0.1}{15+25}$=0.0125mol/L������K=$\frac{1{0}^{-7}��0.0625}{0.0125}$=5��10-7mol•L-1���ʴ�Ϊ��5��10-7mol•L-1��
�۵��μ���25mLʱ���õ���������Ũ�ȵ�HX��NaX�Ļ����Һ����HX�ĵ���Ϊ������Һ�����ԣ���������Ũ�ȴ�СΪ��c��X-����c��Na+����c��H+����c��OH-�����ʴ�Ϊ��c��X-����c��Na+����c��H+����c��OH-����
���� ���⿼�黯ѧƽ��ͼ��ע��ͼ���з�Ӧ���ʵı仯��ƽ���ƶ�����ȷ�¶ȡ�Ũ�ȡ�ѹǿ�Է�Ӧ���ʼ�ƽ���Ӱ�켴�ɽ��ͬʱ��������кͺ���ĵ���ƽ�ⳣ���ļ����Լ�����Ũ�ȴ�С�ıȽϣ��ۺ���ǿ������Ŀ�Ѷ��еȣ�

| A�� | 22.4 L Cl2��ȫ��������������Һʱ��ת�Ƶ�����ΪNA | |
| B�� | �����£�1 L pH=1��H2SO4��Һ�к��е�H+����ĿΪ0.2NA | |
| C�� | ��״���£�2.24 L NH3�к��й��ۼ�����ĿΪ0.3NA | |
| D�� | �����£�22.4 L NO2��N2O4�Ļ�������к���NA����ԭ�� |
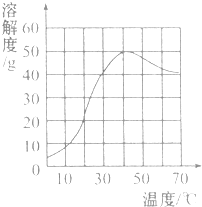
| A�� | �����Ƶ��ܽ�����¶����߶����� | |
| B�� | 30��ʱ�����Ʊ�����Һ����������Ϊ40% | |
| C�� | 40��ʱ�����Ƶ��ܽ��ԼΪ50g | |
| D�� | 60��ʱ�����Ʊ�����Һ���º�һ������ |
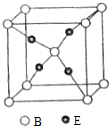
| A�� | ԭ�Ӱ뾶��A��B��C��D��E | |
| B�� | ������AE��CE������ͬ���͵Ļ�ѧ�� | |
| C�� | �����ԣ�D��C | |
| D�� | һ�������£�Ԫ��C��D������������Ӧ��ˮ����֮���ܷ�����Ӧ |
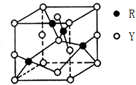 X��Y��Z��RΪǰ������Ԫ�أ�ԭ��������������X��Yͬ���ڣ�X��̬ԭ�ӵ������������Ǵ�����2����Y��̬ԭ�ӵ�s�ܼ���p�ܼ��ϵ�������ȣ�Z�ǵؿ��к�����ߵĽ���Ԫ�أ�R+���ӵ�3d���ȫ������
X��Y��Z��RΪǰ������Ԫ�أ�ԭ��������������X��Yͬ���ڣ�X��̬ԭ�ӵ������������Ǵ�����2����Y��̬ԭ�ӵ�s�ܼ���p�ܼ��ϵ�������ȣ�Z�ǵؿ��к�����ߵĽ���Ԫ�أ�R+���ӵ�3d���ȫ������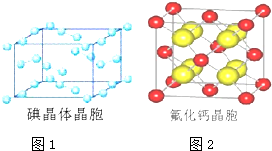
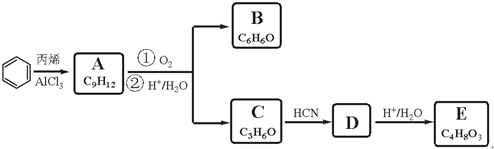
 ��A�ĺ��б�����ͬ���칹�壨��A�⣩��7�֣�
��A�ĺ��б�����ͬ���칹�壨��A�⣩��7�֣�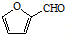 ����һ����Ҫ�Ļ���ԭ�ϣ���ͬ��ȩ�뱽�ӷ�Ӧ���ɷ�ȩ��֬����Ҳ���뱽�ӷ�Ӧ���ɿ�ȩ��֬��д���÷�Ӧ�Ļ�ѧ����ʽ
����һ����Ҫ�Ļ���ԭ�ϣ���ͬ��ȩ�뱽�ӷ�Ӧ���ɷ�ȩ��֬����Ҳ���뱽�ӷ�Ӧ���ɿ�ȩ��֬��д���÷�Ӧ�Ļ�ѧ����ʽ ��
�� G��
G�� ��
��