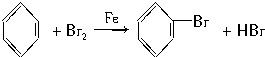
��Ŀ����
12����1������Ľṹʽ��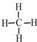 ����ϩ�ĵ���ʽ��
����ϩ�ĵ���ʽ�� ����6.72L����״������ϩ�ͼ���Ļ����ͨ����������ˮ�У���ַ�Ӧ����ˮ������������1.4g��ԭ�����������ϩ�ͼ�������ʵ���֮����1��5
����6.72L����״������ϩ�ͼ���Ļ����ͨ����������ˮ�У���ַ�Ӧ����ˮ������������1.4g��ԭ�����������ϩ�ͼ�������ʵ���֮����1��5��2�������������У�ÿ����һ��̼ԭ�ӣ�1mol������ȫȼ����Ҫ����������������ĸ��B
A��1mol B��1.5mol C��2mol D��2.5mol
��3���Ҵ������������ֲ�ͬ�Ļ�ѧ������ͼ����
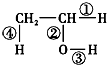 �ڲ�ͬ�Ļ�ѧ��Ӧ�л���Ѳ�ͬ�Ļ�ѧ������д�����з�Ӧ�Ļ�ѧ����ʽ����ָ����Ӧʱ�Ҵ������ж��ѵĻ�ѧ����
�ڲ�ͬ�Ļ�ѧ��Ӧ�л���Ѳ�ͬ�Ļ�ѧ������д�����з�Ӧ�Ļ�ѧ����ʽ����ָ����Ӧʱ�Ҵ������ж��ѵĻ�ѧ����A���Ҵ����ȵ�ͭ˿���·���������Ӧ��2CH3CH2OH+O2 $��_{��}^{Cu}$2CH3CHO+2H2O�����ѣ�����ţ��ٺ͢ۻ�ѧ����
B���Ҵ���Ͷ������ð���ݣ�2C2H5OH+2Na��2C2H5ONa+H2�������ѣ�����ţ��ۻ�ѧ����
���� ��1���ö��ߡ�-�����湲�õ��ӶԼ���ṹʽ����ϩ������̼̼��˫��������̼ʣ��ۼ���H���ͣ��ɴ�д������ʽ����ϩ�ͼ���Ļ����ͨ����������ˮ�У�ֻ����ϩ������ˮ��Ӧ����ַ�Ӧ����ˮ����������1.4gΪ��ϩ������������n=$\frac{m}{M}$���������ʵ���������n=$\frac{V}{{V}_{m}}$������������������ʵ�������������ԭ���������м�������ʵ������ݴ˽��
��2������ͨʽCnH2n+2��ÿ��һ��̼ԭ�ӾͶ�CH2��
��3��A���Ҵ��ڴ�����ʱ��-CH2OH�ṹ������Ϊ-CHO�ṹ�����ݹ����ŵı仯�ж϶��ѵĻ�ѧ����
B���������Ҵ��з�Ӧ����С�������������ݣ����Ƶı������������
��� �⣺��1���������ʽΪCH4��̼ԭ������ԭ��֮���γ�1�Թ��õ��Ӷԣ��ṹʽΪ ����ϩ������̼̼��˫��������̼ʣ��ۼ���H���ͣ��ɴ�д������ʽΪ��
����ϩ������̼̼��˫��������̼ʣ��ۼ���H���ͣ��ɴ�д������ʽΪ�� ����ϩ�����ʵ���Ϊ��n��C2H4��=1.4g��28g•mol-1=0.05mol��������������ʵ���Ϊ��n����ϣ�=6.72L��22.4L•mol-1=0.3mol��n��CH4��=0.3mol-0.05 mol=0.25 mol��n��C2H4����n��CH4��=0.05��0.25=1��5��
����ϩ�����ʵ���Ϊ��n��C2H4��=1.4g��28g•mol-1=0.05mol��������������ʵ���Ϊ��n����ϣ�=6.72L��22.4L•mol-1=0.3mol��n��CH4��=0.3mol-0.05 mol=0.25 mol��n��C2H4����n��CH4��=0.05��0.25=1��5��
�ʴ�Ϊ�� ��
�� ��1��5��
��1��5��
��2������ͨʽCnH2n+2��ÿ��һ��̼ԭ�ӾͶ�CH2��1molCH2��������1.5mol����ѡ��B��
��3��A���Ҵ���Ag������O2��Ӧ������ȩ��ˮ��2CH3CH2OH+O2 $��_{��}^{Cu}$2CH3CHO+2H2O�����Ҵ��ϼ���λ��Ϊ�ٺۣ͢��ʴ�Ϊ��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O���٢ۣ�
B���������Ҵ��з�Ӧ����С�������������ݣ����Ƶı������������Ӧ����ʽΪ��2C2H5OH+2Na��2C2H5ONa+H2�������Ѣۣ��ʴ�Ϊ��2C2H5OH+2Na��2C2H5ONa+H2�����ۣ�
���� ���⿼���Ҵ������ʣ��ѶȲ����˽ⷴӦ�Ļ����ǽ���Ĺؼ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��pH��ֽ���������ˮ��pHΪ2 | |
| B�� | �к͵ζ�ʵ���У��ζ�����Һ���Ӧ�Ŀ̶���0 mL��Ϊ10 mL | |
| C�� | ijͬѧ��������ƽ���̷���5g���룬����λ��0.5g����������ҩƷ����Ϊ5.5g | |
| D�� | ��������Ϊ20%��NaCl��Һ��ˮ��������ϣ�ϡ�ͺ���Һ������������Ϊ10% |
| A�� | 0��1��12��11 | B�� | 14��13��12��11 | C�� | 14��13��2��3 | D�� | 1��10��100��1000 |
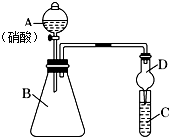 ѧϰ��Ԫ�������ɵ��й�֪ʶ��ijͬѧ����Ԫ�طǽ��������Ӧ��ۺ�����֮��Ĺ�ϵ��ѡ���ɶ�����Ԫ����ɵĻ������������ͼ1װ����һ�������ͬ�����ͬ����Ԫ�طǽ�����ǿ���Ƚϵ�ʵ���о���
ѧϰ��Ԫ�������ɵ��й�֪ʶ��ijͬѧ����Ԫ�طǽ��������Ӧ��ۺ�����֮��Ĺ�ϵ��ѡ���ɶ�����Ԫ����ɵĻ������������ͼ1װ����һ�������ͬ�����ͬ����Ԫ�طǽ�����ǿ���Ƚϵ�ʵ���о���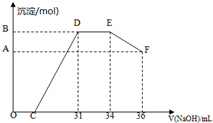 ���ȷ�Ӧʵ�����£�������Բ����ֽ�ֱ��۵���©��״������һ���Ƚ������ڲ��©���ײ���һ��С�ף���ˮ��ʪ������������̨����Ȧ�ϣ��������ʢɳ�������������ij��ɵ���������ĩ�����ۻ�Ͼ��ȣ�����ֽ©���У��������������ز��ڻ�����м��һ��þ������Сľ����ȼþ������ȼ�����������۵Ļ�����Ӧ���ҽ��У�����ҫ�۵�ǿ�⣬����������������
���ȷ�Ӧʵ�����£�������Բ����ֽ�ֱ��۵���©��״������һ���Ƚ������ڲ��©���ײ���һ��С�ף���ˮ��ʪ������������̨����Ȧ�ϣ��������ʢɳ�������������ij��ɵ���������ĩ�����ۻ�Ͼ��ȣ�����ֽ©���У��������������ز��ڻ�����м��һ��þ������Сľ����ȼþ������ȼ�����������۵Ļ�����Ӧ���ҽ��У�����ҫ�۵�ǿ�⣬����������������
 ��
��
 ʵ������Ҫ0.1mol/LNaOH��Һ500mL������������Һ����������ش��������⣮
ʵ������Ҫ0.1mol/LNaOH��Һ500mL������������Һ����������ش��������⣮