��Ŀ����
����Ŀ������(P4)��һ�ֳ����ľ���,�������Ʊ��ϴ������ᡣ
��1��������________(��ԭ�ӻ����)���壬31 g������������������ȫȼ���ͷų�745.5 kJ����������д������ȼ�յ��Ȼ�ѧ��Ӧ����ʽ��______________________________________��
��2����֪����������Һ�ɷ������·�Ӧ��________P4+___HClO3+___ ______��____HCl+____H3PO4,��ƽ�����������Ӧ����ʽ______________���÷�Ӧ����������___________��
��3�������ж���ʵ���ҿɲ���CuSO4��Һ���д������䷴ӦΪ��11P4��60CuSO4��96H2O==20Cu3P��24H3PO4��60H2SO4���÷�Ӧ������������________������11 mol P4��Ӧ������________ mol����ת�ơ�
���𰸡����� P4(s) + 5O2(g) = 2P2O5(s) ��H= -2982.0kJ/mol 3 10 18 H2O 10 12 HClO3 H3PO4 120
��������
��1������Ϊ���Ӿ��壬�����ʽΪP4��31g������������������ȫȼ������P2O5���壬�ͷų�745.5kJ����������1mol������ȫȼ�շų�������=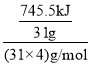 =2982kJ��������ȼ�յ��Ȼ�ѧ��Ӧ����ʽΪ��P4��s��+5O2��g��=2P2O5��s������H=-2982 kJ/mol��
=2982kJ��������ȼ�յ��Ȼ�ѧ��Ӧ����ʽΪ��P4��s��+5O2��g��=2P2O5��s������H=-2982 kJ/mol��
�ʴ�Ϊ�����ӣ�P4��s��+5O2��g��=2P2O5��s������H=-2982 kJ/mol��
��2����Ӧ��PԪ�صĻ��ϼ���0�����ߵ�+5�ۣ�P4Ϊ��ԭ����ClԪ�صĻ��ϼ���+5�۽��͵�-1�ۣ�HClO3Ϊ�����������ݵ�ʧ������Ŀ��ȿ�֪��������֮��Ϊ3��10�����������غ㶨�ɿ�֪ƽ���Ļ�ѧ����ʽΪ3P4+10HClO3+18H2O=10HCl+12H3PO4��
�ʴ�Ϊ��3��10��18H2O��10��12��HClO3
��3���÷�Ӧ�У�CuԪ�صĻ��ϼ���+2�۽��͵�+1�ۣ�������Ԫ����0�۽��͵�-3�ۣ�������Ԫ����0�����ߵ�+5�ۣ���������������H3PO4���÷�Ӧ����11mol���ײμӷ�Ӧ����ת�Ƶ��ӵ����ʵ���=24����5-0��mol=120mol��
�ʴ�Ϊ��H3PO4��120��

 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�