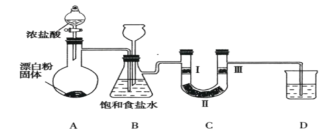
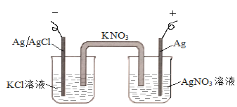
��Ŀ����
����Ŀ�����и�������У�������һ��������ͬ��Ԫ��ԭ�ӵ���(����)
A.3p�ܼ���һ���չ���Ļ�̬ԭ�Ӻͺ�������Ų�Ϊ1s22s22p63s23p2��ԭ��
B.M��ȫ������N��Ϊ4s2��ԭ�Ӻͺ�������Ų�Ϊ1s22s22p63s23p63d64s2��ԭ��
C.�����������Ǻ������������![]() ��ԭ�Ӻͼ۵����Ų�Ϊ4s24p5��ԭ��
��ԭ�Ӻͼ۵����Ų�Ϊ4s24p5��ԭ��
D.2p�ܼ���һ��δ�ɶԵ��ӵĻ�̬ԭ�Ӻͼ۵����Ų�Ϊ2s22p5��ԭ��
���𰸡�B
��������
A.3p�ܼ���һ���չ���Ļ�̬ԭ�Ӻͺ�������Ų�Ϊ1s22s22p63s23p2��ԭ�Ӷ���ָSiԭ�ӣ��ʲ�ѡA��
B. M��ȫ������N��Ϊ4s2��ԭ����Znԭ�ӣ���������Ų�Ϊ1s22s22p63s23p63d64s2��ԭ����Feԭ�ӣ���ѡB��
C. �����������Ǻ������������![]() ��ԭ����Br��Brԭ�Ӻ�������Ų�Ϊ1s22s22p63s23p63d104s24p5���۵����Ų�ʽΪ4s24p5������ͬ��Ԫ��ԭ�ӣ��ʲ�ѡC��
��ԭ����Br��Brԭ�Ӻ�������Ų�Ϊ1s22s22p63s23p63d104s24p5���۵����Ų�ʽΪ4s24p5������ͬ��Ԫ��ԭ�ӣ��ʲ�ѡC��
D. 2p�ܼ���һ��δ�ɶԵ��ӵĻ�̬ԭ�ӿ�����B��F���۵����Ų�Ϊ2s22p5��ԭ����F���ʲ�ѡD��
�ʴ�ѡB��

����Ŀ����ͼΪԪ�����ڱ���һ���֣�
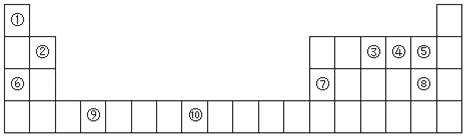
I��(1)Ԫ�آ۵Ļ�̬ԭ�ӹ����ʾʽΪ_________________��
(2)Ԫ�آۢܢݵĵ�һ�������ɴ�С��˳��Ϊ___________����Ԫ�ط��ţ���
(3)������ϵ�ԭ�Ӽ䷴Ӧ�������γ����Ӽ�����_____��ѡ��A��B��C��D����ͬ����
A���ں͢� B���͢� C���ݺ͢� D���͢�
(4)ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ����ϱ���Ԫ�آ���Ԫ�آڵĵ��ʼ��仯���������Ƶ����ʣ�д��Ԫ�آڵ������������NaOH��Һ��Ӧ�����ӷ���ʽ_________________________________��
II��(1)��ͼ�Dz���Ԫ��ԭ�ӵĵ�һ������I1��ԭ�������仯��ϵ������12����17��Ԫ�ص��й�����ȱʧ����
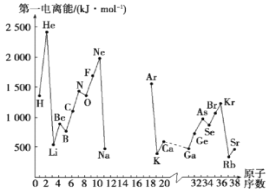
�ٸ���ͬͼʾ�仯���ɣ����Ʋ�S�ĵ�һ�����ܵĴ�С����С����ΧΪ___< S <___ ����Ԫ�ط��ţ�
��ͼ�е�һ��������С��Ԫ�������ڱ��е�λ�� ____________________
(2)��In��ʾԪ�صĵ�n�����ܣ���ͼ�е�a��b��c�ֱ������__________��
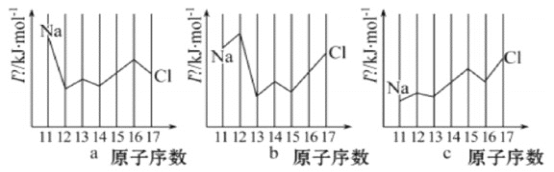
A��aΪI1��bΪI2��cΪI3 B��aΪI3��bΪI2��cΪI1
C��aΪI2��bΪI3��cΪI1 D��aΪI1��bΪI3��cΪI2
(3)ͭ��п����Ԫ�صĵ�һ�����ܡ��ڶ������������ʾ
������ | I1 | I2 |
Cu | 746 | 1958 |
Zn | 906 | 1733 |
ͭ�ĵ�һ������(I1)С��п�ĵ�һ�����ܣ���ͭ�ĵڶ�������(I2)ȴ����п�ĵڶ������ܣ�����Ҫԭ����______________________________________________________